Lighting. Artificial light is generally produced by raising some body to a high temperature. If the temperature of a solid body be greater than that of surrounding bodies it parts with some of its energy in the form of radiation. Whilst the temperature is low these radiations are not of a kind to which the eye is sensitive; they are exclusively radiations less refrangible and of greater wave-length than red light, and may be called infra-red. As the temperature is increased the infra-red radiations increase, but presently there are added radiations which the eye perceives as red light. As the temperature is further increased, the red light increases, and yellow, green and blue rays are successively thrown off. On raising the temperature to a still higher point, radiations of a wave-length shorter even than violet light are produced, to which the eye is insensitive, but which act strongly on certain chemical substances; these may be called ultra-violet rays. Thus a very hot body in general throws out rays of various wave-length; the hotter the body the more of every kind of radiation will it throw out, but the proportion of short waves to long waves becomes vastly greater as the temperature is increased. Our eyes are only sensitive to certain of these waves, viz. those not very long and not very short. The problem of the artificial production of light with economy of energy is the same as that of raising some body to such a temperature that it shall give as large a proportion as possible of those rays which the eye is capable of feeling. For practical purposes this temperature is the highest temperature we can produce. As an illustration of the luminous effect of the high temperature produced by converting other forms of energy into heat within a small space, consider the following statements. If burned in ordinary gas burners, 120 cub. ft. of 15 candle gas will give a light of 360 standard candles for one hour. The heat produced by the combustion is equivalent to about 60 million foot-pounds. If this gas be burned in a modern gas-engine, about 8 million foot-pounds of useful work will be done outside the engine, or about 4 horse-power for one hour. If this be used to drive a dynamo for one hour, even if the machine has an efficiency of only 80%, the energy of the current will be about 6,400,000 foot-pounds per hour, about half of which, or only 3,200,000 foot-pounds, is converted into radiant energy in the electric arc. But this electric arc will radiate a light of 2000 candles when viewed horizontally, and two or three times as much when viewed from below. Hence 3 million foot-pounds changed to heat in the electric arc may be said roughly to affect our eyes six times as much as 60 million foot-pounds changed to heat in an ordinary gas burner.
Owing to the high temperature at which it remains solid, and to its great emissive power, the radiant body used for artificial illumination is usually some form of carbon. In an oil or ordinary coal-gas flame this carbon is present in minute particles derived from the organic substances with which the flame is supplied and heated to incandescence by the heat liberated in their decomposition, while in the electric light the incandescence is the effect of the heat developed by the electric current passed through a resisting rod or filament of carbon. In some cases, however, other substances replace carbon as the radiating body; in the incandescent gas light certain earthy oxides are utilized, and in metallic filament electric lamps such metals as tungsten or tantalum.
1. Oil Lighting
From the earliest times the burning of oil has been a source of light, but until the middle of the 19th century only oils of vegetable and animal origin were employed in indoor lamps for this purpose. Although many kinds were Vegetable and animal oils. used locally, only colza and sperm oils had any very extended use, and they have been practically supplanted by mineral oil, which was introduced as an illuminant in 1853. Up to the latter half of the 18th century the lamps were shallow vessels into which a short length of wick dipped; the flame was smoky and discharged acrid vapours, giving the minimum of light with the maximum of smell. The first notable improvement was made by Ami Argand in 1784. His burner consisted of two concentric tubes between which the tubular wick was placed; the open inner tube led a current of air to play upon the inner surface of the circular flame, whilst the combustion was materially improved by placing around the flame a chimney which rested on a perforated gallery a short distance below the burner. Argand’s original burner is the parent form of innumerable modifications, all more or less complex, such as the Carcel and the moderator.
 |
| Fig. 1. |
 |
| Fig. 2.—Section of Reading Lamp. |
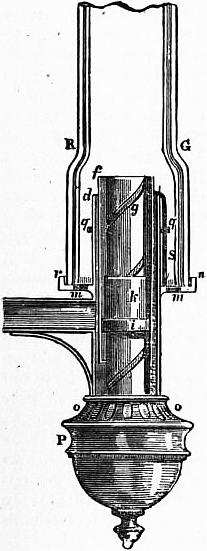
A typical example of the Argand burner and chimney is represented in fig. 1, in which the burner is composed of three tubes, d, f, g. The tube g is soldered to the bottom of the tube d, just above o, and the interval between the outer surface of the tube g and the inner surface of the tube d is an annular cylindrical cavity closed at the bottom, containing the cylindrical cotton wick immersed in oil. The wick is fixed to the wick tube ki, which is capable of being moved spirally; within the annular cavity is also the tube f, which can be moved round, and serves to elevate and depress the wick. P is a cup that screws on the bottom of the tube d, and receives the superfluous oil that drops down from the wick along the inner surface of the tube g. The air enters through the holes o, o, and passes up through the tube g to maintain the combustion in the interior of the circular flame. The air which maintains the combustion on the exterior part of the wick enters through the holes m, with which rn is perforated. When the air in the chimney is rarefied by the heat of the flame, the surrounding heavier air, entering the lower part of the chimney, passes upward with a rapid current, to restore the equilibrium. RG is the cylindrical glass chimney with a shoulder or constriction at R, G. The oil flows from a side reservoir, and occupies the cavity between the tubes g and d. The part ki is a short tube, which receives the circular wick, and slides spirally on the tube g, by means of a pin working in the hollow spiral groove on the exterior surface of g. The wick-tube has also a catch, which works in a perpendicular slit in the tube f; and, by turning the tube f, the wick-tube will be raised or lowered, for which purpose a ring, or gallery, rn, fits on the tube d, and receives the glass chimney RG; a wire S is attached to the tube f, and, bending over, descends along the outside of d. The part rn, that supports the glass chimney, is connected by four other wires with the ring q, which surrounds the tube d, and can be moved round. When rn is turned round, it carries with it the ring q, the wire S, and the tube f, thus raising or depressing the wick.
A device in the form of a small metallic disk or button, known as the Liverpool button from having been first adopted in the so-called Liverpool lamp, effects for the current of air passing up the interior of the Argand burner the same object as the constriction of the chimney RG secures in the case of the external tube. The button fixed on the end of a wire is placed right above the burner tube g, and throws out equally all round against the flame the current of air which passes up through g. The result of these expedients, when properly applied, is the production of an exceedingly solid brilliant white light, absolutely smokeless, this showing that the combustion of the oil is perfectly accomplished.
The means by which a uniformly regulated supply of oil is brought to the burner varies with the position of the oil reservoir. In some lamps, not now in use, by ring-formed reservoirs and other expedients, the whole of the oil was kept as nearly as possible at the level of the burner. In what are termed fountain reading, or study lamps, the principal reservoir is above the burner level, and various means are adopted for maintaining a supply from them at the level of the burner. But the most convenient position for the oil reservoir in lamps for general use is directly under the burner, and in this case the stand of the lamp itself is utilized as the oil vessel. In the case of fixed oils, as the oils of animal and vegetable origin used to be called, it is necessary with such lamps to introduce some appliance for forcing a supply of oil to the burner, and many methods of effecting this were devised, most of which were ultimately superseded by the moderator lamp. The Carcel or pump lamp, invented by B. G. Carcel in 1800, is still to some extent used in France. It consists of a double piston or pump, forcing the oil through a tube to the burner, worked by clockwork.
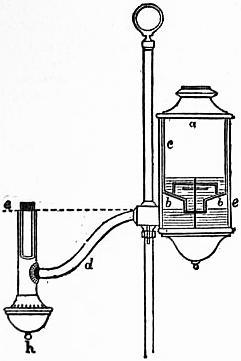
A form of reading lamp still in use is seen in section in fig. 2. The lamp is mounted on a standard on which it can be raised or lowered at will, and fixed by a thumb screw. The oil reservoir is in two parts, the upper ac being an inverted flask which fits into bb, from which the burner is directly fed through the tube d; h is an overflow cup for any oil that escapes at the burner, and it is pierced with air-holes for admitting the current of air to the centre tube of the Argand burner. The lamp is filled with oil by withdrawing the flask ac, filling it, and inverting it into its place. The under reservoir bb fills from it to the burner level ee, on a line with the mouth of ac. So soon as that level falls below the mouth of ac, a bubble of air gets access to the upper reservoir, and oil again fills up bb to the level ee.
 |
| Fig. 3.—Section of Moderator Lamp. |
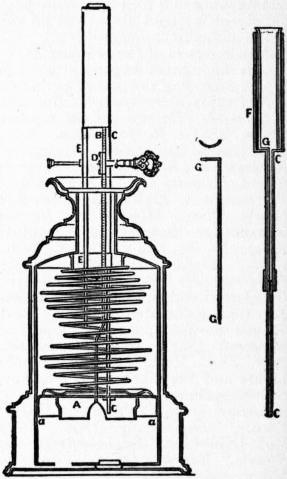
The moderator lamp (fig. 3), invented by Franchot about 1836, from the simplicity and efficiency of its arrangements rapidly superseded almost all other forms of mechanical lamp for use with animal and vegetable oils. The two essential features of the moderator lamp are (1) the strong spiral spring which, acting on a piston within the cylindrical reservoir of the lamp, serves to propel the oil to the burner, and (2) the ascending tube C through which the oil passes upwards to the burner. The latter consist of two sections, the lower fixed to and passing through the piston A into the oil reservoir, and the upper attached to the burner. The lower or piston section moves within the upper, which forms a sheath enclosing nearly its whole length when the spring is fully wound up. Down the centre of the upper tube passes a wire, “the moderator,” G, and it is by this wire that the supply of oil to the burner is regulated. The spring exerts its greatest force on the oil in the reservoir when it is fully wound up, and in proportion as it expands and descends its power decreases. But when the apparatus is wound up the wire passing down the upper tube extends throughout the whole length of the lower and narrower piston tube, obstructing to a certain extent the free flow of the oil. In proportion as the spring uncoils, the length of the wire within the lower tube is decreased; the upward flow of oil is facilitated in the same ratio as the force urging it upwards is weakened. In all mechanical lamps the flow is in excess of the consuming capacity of the burner, and in the moderator the surplus oil, flowing over the wick, falls back into the reservoir above the piston, whence along with new supply oil it descends into the lower side by means of leather valves a, a. B represents the rack which, with the pinion D, winds up the spiral spring hard against E when the lamp is prepared for use. The moderator wire is seen separately in GG; and FGC illustrates the arrangement of the sheathing tubes, in the upper section of which the moderator is fixed.
As early as 1781 the idea was mooted of burning naphtha, obtained by the distillation of coal at low temperatures, for illuminating purposes, and in 1820, when coal gas was struggling into prominence, light oils obtained Mineral oils. by the distillation of coal tar were employed in the Holliday lamp, which is still the chief factor in illuminating the street barrow of the costermonger. In this lamp the coal naphtha is in a conical reservoir, from the apex of which it flows slowly down through a long metal capillary to a rose burner, which, heated up by the flame, vaporizes the naphtha, and thus feeds the ring of small jets of flame escaping from its circumference.
It was in 1847 that James Young had his attention drawn to an exudation of petroleum in the Riddings Colliery at Alfreton, in Derbyshire, and found that he could by distillation obtain from it a lubricant of considerable value. The commercial success of this material was accompanied by a failure of the supply, and, rightly imagining that as the oil had apparently come from the Coal Measures, it might be obtained by distillation from material of the same character, Young began investigations in this direction, and in 1850 started distilling oils from a shale known as the “Bathgate mineral,” in this way founding the Scotch oil industry. At first little attention was paid to the fitness of the oil for burning purposes, although in the early days at Alfreton Young attempted to burn some of the lighter distillates in an Argand lamp, and later in a lamp made many years before for the consumption of turpentine. About 1853, however, it was noticed that the lighter distillates were being shipped to Germany, where lamps fitted for the consumption of the grades of oil now known as lamp oil were being made by Stohwasser of Berlin; some of these lamps were imported, and similar lamps were afterwards manufactured by Laidlaw in Edinburgh.
In Pennsylvania in 1859 Colonel E. L. Drake’s successful boring for petroleum resulted in the flooding of the market with oil at prices never before deemed possible, and led to the introduction of lamps from Germany for its consumption. Although the first American patent for a petroleum lamp is dated 1859, that year saw forty other applications, and for the next twenty years they averaged about eighty a year.
English lamp-makers were not behind in their attempts to improve on the methods in use for producing the highest results from the various grades of oil, and in 1865 Hinks introduced the duplex burner, while later improvements made in various directions, by Hinks, Silber, and Defries led to the high degree of perfection to be found in the lamps of to-day. Mineral oil for lamps as used in England at the present time may be defined as consisting of those portions of the distillate from shale oil or crude petroleum which have their flash-point above 73° F., and which are mobile enough to be fed by capillarity in sufficient quantity to the flame. The oil placed in the lamp reservoir is drawn up by the capillarity of the wick to the flame, and being there volatilized, is converted by the heat of the burning flame into a gaseous mixture of hydrogen and hydrocarbons, which is ultimately consumed by the oxygen of the air and converted into carbon dioxide and water vapour, the products of complete combustion.
To secure high illuminating power, together with a smokeless flame and only products of complete combustion, strict attention must be paid to several important factors. In the first place, the wick must be so arranged as to supply the right quantity of oil for gasification at the burner-head—the flame must be neither starved nor overfed: if the former is the case great loss of light is occasioned, while an excess of oil, by providing more hydrocarbons than the air-supply to the flame can completely burn, gives rise to smoke and products of incomplete combustion. The action of the wick depending on the capillary action of the microscopic tubes forming the cotton fibre, nothing but long-staple cotton of good quality should be employed; this should be spun into a coarse loose thread with as little twist in it as possible, and from this the wick is built up. Having obtained a wick of soft texture and loose plait, it should be well dried before the fire, and when put in position in the lamp must fill the wick-holder without being compressed. It should be of sufficient length to reach to the bottom of the oil reservoir and leave an inch or two on the bottom. Such a wick will suck up the oil in a regular and uniform way, provided that the level of the oil is not allowed to fall too low in the lamp, but it must be remembered that the wick acts as a filter for the oil, and that if any sediment be present it will be retained by and choke the capillaries upon which the action of the wick depends, so that a wick should not be used for too long a time. A good rule is that the wick should, when new, trail for 2 in. on the bottom of the oil vessel, and should be discarded when these 2 in. have been burnt off.
When the lamp is lighted the oil burns with a heavy, smoky flame, because it is not able to obtain sufficient oxygen to complete the combustion, and not only are soot flakes produced, but products of incomplete combustion, such as carbon monoxide and even petroleum vapour, escape—the first named highly injurious to health, and the second of an offensive odour. To supply the necessary amount of air to the flame, an artificial draught has to be created which shall impinge upon the bottom of the flame and sweep upwards over its surface, giving it rigidity, and by completing the combustion in a shorter period of time than could be done otherwise, increasing the calorific intensity and thus raising the carbon particles in the flame to a far higher incandescence so as to secure a greater illuminating power. This in practice has been done in two ways, first by drawing in the air by the up-suck of the heated and expanded products of combustion in a chimney fitted over the flame, and secondly by creating a draught from a small clockwork fan in the base of the lamp. It is necessary to break the initial rush of the draught: this is mostly effected by disks of perforated metal in the base of the burner, called diffusers, while the metal dome which surrounds and rises slightly above the wick-holder serves to deflect the air on to the flame, as in the Wanzer lamp. These arrangements also act to a certain extent as regenerators, the air passing over the heated metal surfaces being warmed before reaching the flame, whilst disks, cones, buttons, perforated tubes, inner air-tubes, &c., have been introduced to increase the illuminating power and complete the combustion.
Table I.
| Type. | Name. | Grains of Oil per candle-power per hour. |
Total Candle-power. | ||
| American. | Russian. | American. | Russian. | ||
| Circular wick | Veritas, 60-line | 64.5 | 112.5 | 122.5 | 78 |
| Veritas, 30-line | 42.5 | 50. | 60 | 60 | |
| Veritas, 20-line | 43.75 | 58.5 | 40 | 35 | |
| Ariel, 12-line center draught | 52.8 | 70.9 | 18 | 18 | |
| Reading, 14-line | 97.9 | 85.4 | 12 | 12 | |
| Kosmos, 10-line | 63.9 | 97.2 | 9 | 9 | |
| Wizard, 15-line | 56.9 | 51.3 | 18 | 19 | |
| Flat wick, single | Wanzer, no glass | 42.6 | 48.3 | 17 | 17 |
| Solid slip, gauze and cone | 84.4 | 84.4 | 8 | 8 | |
| Old slip, fixed gauze | 60.9 | 89.3 | 7 | 7 | |
| Flat wick, duplex | Feeder wick | 56.2 | 55.7 | 20 | 22 |
| Ordinary | 51.2 | 46.6 | 20 | 22 | |
| American oil—Sp. gr. 0.7904; flash-point, 110°F. Russian oil—Sp. gr. 0.823; flash-point, 83° F. | |||||
According to Sir Boverton Redwood, duplex burners which give a flame of 28 candle-power have an average oil consumption of 50 grains per candle per hour, while Argand flames of 38 candle-power consume about 45 grains of oil per candle per hour. These figures were obtained from lamps of the best types, and to obtain information as to the efficiency of the lamps used in daily practice, a number of the most popular types were examined, using both American and Russian oil. The results obtained are embodied in Table 1. The first noteworthy point in this table is the apparent superiority of the American over Russian oil in the majority of the lamps employed, and there is no doubt that the bulk of the lamps on the market are constructed to burn American or shale oil. A second interesting point is that with the flat-flame lamps the Russian oil is as good as the American. We have Redwood’s authority, moreover, for the fact that after prolonged burning the Russian oil, even in lamps least suited to it, gives highly improved results. Although the average consumption with these lamps is close upon 60 grains per candle with American oil, yet some of the burners are so manifestly wasteful that 50 grains per candle-power per hour is the fairest basis to take for any calculation as to cost.
The dangers of the mineral oil lamp, which were a grave drawback in the past, have been very much reduced by improvements in construction and quality, and if it were possible to abolish the cheap and dangerous rubbish sold in poor neighbourhoods, and to prevent the use of side-fillers and glass reservoirs in lamps of better quality, a still larger reduction in the number of accidents would take place. In the use of the lamp for domestic purposes only soft well-fitting wicks should be employed, and the lamp should be filled with oil each day so as never to allow it to burn too low and so leave a large space above the surface of the oil in the reservoir. The lamp should never be moved whilst alight, and it should only be put out by means of a proper extinguisher or by blowing across the top instead of down the chimney. By these means the risk of accident would be so reduced as to compare favourably with other illuminants.
Candles, oil and coal gas all emit the same products of complete combustion, viz. carbon dioxide and water vapour. The quantities of these compounds emitted from different illuminants for every candle of light per hour will be seen from the following table:
| Cubic Feet per Candle. | ||
| Illuminant. | Carbon Dioxide. | Water Vapour. |
| Sperm candle | 0.41 | 0.41 |
| Oil lamp | 0.24 | 0.18 |
| Gas—Flat flame | 0.26 | 0.67 |
| Argand | 0.17 | 0.45 |
| Regenerative | 0.07 | 0.19 |
| Incandescent | 0.03 | 0.08 |
From these data it appears that if the sanitary condition of the air of a dwelling-room be measured by the amount of carbon dioxide present, as is usually done, candles are the most prejudicial to health and comfort, oil lamps less so, and gas least, an assumption which practical experience does not bear out. The explanation of this is to be found in these facts: First, where we illuminate a room with candles or oil we are contented with a less intense and more local light than when we are using gas, and in a room of ordinary size would be more likely to use a lamp or two candles than the far higher illumination we should demand if gas were employed. Secondly, the amount of water vapour given off during the combustion of gas is greater than in the case of the other illuminants, and water vapour absorbing radiant heat from the burning gas becomes heated, and, diffusing itself about the room, causes great oppression. Also the air, being highly charged with moisture, is unable to take up so rapidly the water vapour which is always evaporating from the surface of our skin, and in this way the functions of the body receive a slight check, resulting in a feeling of depression.
A very successful type of oil lamp for use in engineering is represented by the Lucigen, Doty, and Wells lights, in which the oil is forced from a reservoir by air-pressure through a spiral heated by the flame of the lamp, and the heated Oil-spray lamps. oil, being then ejected partly as vapour and partly as spray, burns with a large and highly luminous flame. The great drawback to these devices is that a certain proportion of the oil spray escapes combustion and is deposited in the vicinity of the light. This form of lamp is often used for heating as well as lighting; the rivets needed for the Forth Bridge were heated in trays by lamps of this type at the spot where they were required. The great advantage of these lamps was that oils of little value could be employed, and the light obtained approximated to 750 candles per gallon of oil consumed. They may to a certain extent be looked upon as the forerunners of perhaps the most successful form of incandescent oil-burner.
As early as 1885 Arthur Kitson attempted to make a burner for heating purposes on the foregoing principle, i.e. by injecting oil under pressure from a fine tube into a chamber where it would be heated by the waste heat escaping Oil applied to incandescent lighting. from the flame below, the vapour so produced being made to issue from a small jet under the pressure caused by the initial air-pressure and the expansion in the gasifying tube. This jet of gas was then led into what was practically an atmospheric burner, and drew in with it sufficient air to cause its combustion with a non-luminous blue flame of great heating power. At the time when this was first done the Welsbach mantle had not yet reached the period of commercial utility, and attempts were made to use this flame for the generation of light by consuming it in a mantle of fine platinum gauze, which, although giving a very fine illuminating effect during the first few hours, very soon shared the fate of all platinum mantles—that is, carbonization of the platinum surface took place, and destroyed its power of light emissivity. It was not until 1893 that the perfecting of the Welsbach mantle enabled this method of consuming the oil to be employed. The Kitson lamp, and also the Empire lamp on a similar principle, have given results which ought to ensure their future success, the only drawback being that they need a certain amount of intelligent care to keep them in good working order.
Oil gas and oil vapours differ from coal gas merely in the larger proportion and greater complexity of the hydrocarbon molecules present, and to render the oil flame available for incandescent lighting it is only necessary to Incandescent table-lamps. cause the oil gas or vapour to become mixed with a sufficient proportion of air before it arrives at the point of combustion. But with gases so rich in hydrocarbons as those developed from oil it is excessively difficult to get the necessary air intimately and evenly mixed with the gas in sufficient proportion to bring about the desired result. If even coal gas be taken and mixed with 2.27 volumes of air, its luminosity is destroyed, but such a flame would be useless with the incandescent mantle, as if the non-luminous flame be superheated a certain proportion of its luminosity will reappear. When such a flame is used with a mantle the superheating effect of the mantle itself very quickly leads to the decomposition of the hydrocarbons and blackening of the mantle, which not only robs it of its light-giving powers, but also rapidly ends its life. If, however, the proportion of air be increased, the appearance of the flame becomes considerably altered, and the hydrocarbon molecules being burnt up before impact with the heated surface of the mantle, all chance of blackening is avoided.
On the first attempts to construct a satisfactory oil lamp which could be used with the incandescent mantle, this trouble showed itself to be a most serious one, as although it was comparatively easy so to regulate a circular-wicked flame fed by an excess of air as to make it non-luminous, the moment the mantle was put upon this, blackening quickly appeared, while when methods for obtaining a further air supply were devised, the difficulty of producing a flame which would burn for a considerable time without constant necessity for regulation proved a serious drawback. This trouble has militated against most of the incandescent oil lamps placed upon the market.
It soon became evident that if a wick were employed the difficulty of getting it perfectly symmetrical was a serious matter, and that it could only be utilized in drawing the oil up to a heating chamber where it could be volatilized to produce the oil gas, which on then being mixed with air would give the non-luminous flame. In the earlier forms of incandescent oil lamps the general idea was to suck the oil up by the capillarity of a circular wick to a point a short distance below the opening of the burner at which the flame was formed, and here the oil was vaporized or gasified by the heat of the head of the burner. An air supply was then drawn up through a tube passing through the centre of the wick-tube, while a second air current was so arranged as to discharge itself almost horizontally upon the burning gas below the cap, in this way giving a non-luminous and very hot flame, which if kept very carefully adjusted afforded excellent results with an incandescent mantle. It was an arrangement somewhat of this character that was introduced by the Welsbach Company. The lamps, however, required such careful attention, and were moreover so irregular in their performance, that they never proved very successful. Many other forms have reached a certain degree of perfection, but have not so far attained sufficient regularity of action to make them commercial successes. One of the most successful was devised by F. Altmann, in which an ingenious arrangement caused the vaporization of oil and water by the heat of a little oil lamp in a lower and separate chamber, and the mixture of oil gas and steam was then burnt in a burner-head with a special arrangement of air supply, heating a mantle suspended above the burner-head.
The perfect petroleum incandescent lamp has not yet been made, but the results thus obtained show that when the right system has been found a very great increase in the amount of light developed from the petroleum may be expected. In one lamp experimented with for some time it was easy to obtain 3500 candle hours per gallon of oil, or three times the amount of light obtainable from the oil when burnt under ordinary conditions.
Before the manufacture of coal-gas had become so universal as it is at present, a favourite illuminant for country mansions and even villages where no coal-gas was available was a mixture of air with the vapour of very volatile Air-gas. hydrocarbons, which is generally known as “air-gas.” This was produced by passing a current of dry air through or over petroleum spirit or the light hydrocarbons distilled from tar, when sufficient of the hydrocarbon was taken up to give a luminous flame in flat flame and Argand burners in the same way as coal-gas, the trouble being that it was difficult to regulate the amount of hydrocarbon held in suspension by the air, as this varied very widely with the temperature. As coal-gas spread to the smaller villages and electric lighting became utilized in large houses, the use of air-gas died out, but with the general introduction of the incandescent mantle it again came to the front. In the earlier days of this revival, air-gas rich in hydrocarbon vapour was made and was further aerated to give a non-luminous flame by burning it in an atmospheric burner.
One of the best illustrations of this system was the Aerogene gas introduced by A. I. van Vriesland, which was utilized for lighting a number of villages and railway stations on the continent of Europe. In this arrangement a revolving coil of pipes continually dips into petroleum spirit contained in a cylinder, and the air passed into the cylinder through the coil of pipes becomes highly carburetted by the time it reaches the outlet at the far end of the cylinder. The resulting gas when burnt in an ordinary burner gives a luminous flame; it can be used in atmospheric burners differing little from those of the ordinary type. With an ordinary Welsbach “C” burner it gives a duty of about 30 candles per foot of gas consumed, the high illuminating power being due to the fact that the gas is under a pressure of from 6 to 8 in. With such a gas, containing a considerable percentage of hydrocarbon vapour, any leakage into the air of a room would give rise to an explosive mixture, in the same way that coal-gas would do, but inasmuch as mixtures of the vapour of petroleum spirit and air are only explosive for a very short range, that is, from 1.25 to 5.3%, some systems have been introduced in which by keeping the amount of petroleum vapour at 2% and burning the gas under pressure in a specially constructed non-aerating mantle burner, not only has it been found possible to produce a very large volume of gas per gallon of spirit employed, but the gas is itself non-explosive, increase in the amount of air taking it farther away from the explosive limit. The Hooker, De Laitte and several other systems have been based upon this principle.
2. Gas Lighting
In all measurements of illuminating value the standard of comparison used in England is the light yielded by a sperm candle of the size known as “sixes,” i.e. six to the pound, consuming 120 grains of sperm per hour, and although in photometric work slight inequalities in burning have led to the candle being discarded in practice, the standard lamps burning pentane vapour which have replaced them are arranged to yield a light of ten candles, and the photometric results are expressed as before in terms of candles.
When William Murdoch first used coal-gas at his Redruth home in 1779, he burnt the gas as it escaped from the open end of a small iron tube, but soon realizing that this plan entailed very large consumption of gas and gave a very small amount of light, he welded up the end of his tube and bored three small holes in it, so arranged that they formed three divergent jets of flame. From the shape of the flame so produced this burner received the name of the “cockspur” burner, and it was the one used by Murdoch when in 1807 he fitted up an installation of gas lighting at Phillips & Lee’s works in Manchester. This—the earliest form of gas burner—gave an illuminating value of a little under one candle per cubic foot of gas consumed, and this duty was slightly increased when the burner was improved by flattening up the welded end of the tube and making a series of small holes in line and close together, the jets of flame from which gave the burner the name of the “cockscomb.” It did not need much inventive faculty to replace the line of holes by a saw-cut, the gas issuing from which burnt in a sheet, the shape of which led to the burner being called the “batswing.” This was followed in 1820 by the discovery of J. B. Neilson, of Glasgow, whose name is remembered in connexion with the use of the hot-air blast in iron-smelting, that, by allowing two flames to impinge upon one another so as to form a flat flame, a slight increase in luminosity was obtained, and after several preliminary stages the union jet or “fishtail” burner was produced. In this form of burner two holes, bored at the necessary angle in the same nipple, caused two streams of gas to impinge upon each other so that they flattened themselves out into a sheet of flame. The flames given by the batswing and fishtail burners differed in shape, the former being wide and of but little height, whilst the latter was much higher and more narrow. This factor ensured for the fishtail a greater amount of popularity than the batswing burner had obtained, as the flame was less affected by draughts and could be used with a globe, although the illuminating efficiency of the two burners differed little.
In a lecture at the Royal Institution on the 20th of May 1853, Sir Edward Frankland showed a burner he had devised for utilizing the heat of the flame to raise the temperature of the air supply necessary for the combustion Regenerative burner. of the gas. The burner was an Argand of the type then in use, consisting of a metal ring pierced with holes so as to give a circle of small jets, the ring of flame being surrounded by a chimney. But in addition to this chimney, Frankland added a second external one, extending some distance below the first and closed at the bottom by a glass plate fitted air-tight to the pillar carrying the burner. In this way the air needed for the combustion of the gas had to pass down the space between the two chimneys, and in so doing became highly heated, partly by contact with the hot glass, and partly by radiation. Sir Edward Frankland estimated that the temperature of the air reaching the flame was about 500°F. In 1854 a very similar arrangement was brought forward by the Rev. W. R. Bowditch, and, as a large amount of publicity was given to it, the inception of the regenerative burner was generally ascribed to Bowditch, although undoubtedly due to Frankland.
The principle of regeneration was adopted in a number of lamps, the best of which was brought out by Friedrich Siemens in 1879. Although originally made for heating purposes, the light given by the burner was so effective and superior to anything obtained up to that time that it was with some slight alterations adapted for illuminating purposes.
Improvements followed in the construction and design of the regenerative lamp, and when used as an overhead burner it was found that not only was an excellent duty obtained per cubic foot of gas consumed, but that the lamp could be made a most efficient engine of ventilation, as an enormous amount of vitiated air could be withdrawn from the upper part of a room through a flue in the ceiling space. So marked was the increase in light due to the regeneration that a considerable number of burners working on this principle were introduced, some of them like the Wenham and Cromartie coming into extensive use. They were, however, costly to install, so that the flat flame burner retained its popularity in spite of the fact that its duty was comparatively low, owing to the flame being drawn out into a thin sheet and so exposed to the cooling influence of the atmosphere. Almost at the same time that Murdoch was introducing the cockscomb and cockspur burners, he also made rough forms of Argand burner, consisting of two concentric pipes between which the gas was led and burnt with a circular flame. This form was soon improved by filling in the space between the tubes with a ring of metal, bored with fine holes so close together that the jets coalesced in burning and gave a more satisfactory flame, the air necessary to keep the flame steady and ensure complete combustion being obtained by the draught created by a chimney placed around it. When it began to be recognized that the temperature of the flame had a great effect upon the amount of light emitted, the iron tips, which had been universally employed, both in flat flame and Argand burners, were replaced by steatite or other non-conducting material of similar character, to prevent as far as possible heat from being withdrawn from the flame by conduction.
In 1880 the burners in use for coal-gas therefore consisted of flat flame, Argand, and regenerative burners, and the duty given by them with a 16-candle gas was as follows:—
| Burner. | Candle units per cub. ft. of gas. |
| Union jet flat flame, No. 0 | 0.59 |
| ” ” 1 | 0.85 |
| ” ” 2 | 1.22 |
| ” ” 3 | 1.63 |
| ” ” 4 | 1.74 |
| ” ” 5 | 1.87 |
| ” ” 6 | 2.15 |
| ” ” 7 | 2.44 |
| Ordinary Argand | 2.90 |
| Standard Argand | 3.20 |
| Regenerative | 7 to 10 |
The luminosity of a coal-gas flame depends upon the number of carbon particles liberated within it, and the temperature to which they can be heated. Hence the light given by a flame of coal-gas can be augmented by (1) increasing the number of the carbon particles, and (2) raising the temperature to which they are exposed. The first process is carried out by enrichment (see Gas: Manufacture), the second is best obtained by regeneration, the action of which is limited by the power possessed by the material of which burners are composed to withstand the superheating. Although with a perfectly made regenerative burner it might be possible for a short time to get a duty as high as 16 candles per cubic foot from ordinary coal-gas, such a burner constructed of the ordinary materials would last only a few hours, so that for practical use and a reasonable life for the burner 10 candles per cubic foot was about the highest commercial duty that could be reckoned on. This limitation naturally caused inventors to search for methods by which the emission of light could be obtained from coal-gas otherwise than by the incandescence of the carbon particles contained within the flame itself. A coal-gas flame consumed in an atmospheric burner under the conditions necessary to develop its maximum heating power could be utilized to raise to incandescence particles having a higher emissivity for light than carbon. This led to the gradual evolution of incandescent gas lighting.
Long before the birth of the Welsbach mantle it had been known that when certain unburnable refractory substances were heated to a high temperature they emitted light, and Goldsworthy Gurney in 1826 showed that a Incandescent gas light. cylinder of lime could be brought to a state of dazzling brilliancy by the flame of the oxy-hydrogen blowpipe, a fact which was utilized by Thomas Drummond shortly afterwards in connexion with the Ordnance Survey of Ireland. The mass of a lime cylinder is, however, relatively very considerable, and consequently an excessive amount of heat has to be brought to bear upon it, owing to radiation and conduction tending to dissipate the heat. This is seen by holding in the flame of an atmospheric burner a coil of thick platinum wire, the result being that the wire is heated to a dull red only. With wire of medium thickness a bright red heat is soon attained, and a thin wire glows with a vivid incandescence, and will even melt in certain parts of the flame. Attempts were accordingly made to reduce the mass of the material heated, and this form of lighting was tried in the streets of Paris, buttons of zirconia and magnesia being heated by an oxy-coal-gas flame, but the attempt was soon abandoned owing to the high cost and constant renewals needed. In 1835 W. H. Fox Talbot discovered that even the feeble flame of a spirit lamp is sufficient to heat lime to incandescence, provided the lime be in a sufficiently fine state of division. This condition he fulfilled by soaking blotting-paper in a solution of a calcium salt and then incinerating it. Up to 1848, when J. P. Gillard introduced the intermittent process of making water-gas, the spirit flame and oxy-hydrogen flame were alone free from carbon particles. Desiring to use the water-gas for lighting as well as heating purposes Gillard made a mantle of fine platinum gauze to fit over the flame, and for a time obtained excellent results, but after a few days the lighting value of the mantle fell away gradually until it became useless, owing to the wire becoming eroded on the surface by the flame gases. This idea has been revived at intervals, but the trouble of erosion has always led to failure.
The next important stage in the history of gas lighting was the discovery by R. W. von Bunsen about 1855 of the atmospheric burner, in which a non-luminous coal-gas flame is obtained by causing the coal-gas before its combustion to mix with a certain amount of air. This simple appliance has opened up for coal-gas a sphere of usefulness for heating purposes as important as its use for lighting. After the introduction of the atmospheric burner the idea of the incandescent mantle was revived early in the eighties by the Clamond basket and a resuscitation of the platinum mantle. The Clamond basket or mantle, as shown at the Crystal Palace exhibition of 1882-1883, consisted of a cone of threads of calcined magnesia. A mixture of magnesium hydrate and acetate, converted into a paste or cream by means of water, was pressed through holes in a plate so as to form threads, and these, after being moulded to the required shape, were ignited. The heat decomposed the acetate to form a luting material which glued the particles of magnesium oxide produced into a solid mass, whilst the hydrate gave off water and became oxide. The basket was supported with its apex downwards in a little platinum wire cage, and a mixture of coal-gas and air was driven into it under pressure from an inverted blowpipe burner above it.
The Welsbach mantle was suggested by the fact that Auer von Welsbach had been carrying out researches on the rare earths, with constant use of the spectroscope. Desiring to obtain a better effect than that produced by heating his material on a platinum wire, he immersed cotton in a solution of the metallic salt, and after burning off the organic matter found that a replica of the original thread, composed of the oxide of the metal, was left, and that it glowed brightly in the flame. From this he evolved the idea of utilizing a fabric of cotton soaked in a solution of a metallic salt for lighting purposes, and in 1885 he patented his first commercial mantle. The oxides used in these mantles were zirconia, lanthania, and yttria, but these were so fragile as to be practically useless, whilst the light they emitted was very poor. Later he found that the oxide of thorium—thoria—in conjunction with other rare earth oxides, not only increased the light-giving powers of the mantle, but added considerably to its strength, and the use of this oxide was protected by his 1886 patent. Even these mantles were very unsatisfactory until it was found that the purity of the oxides had a wonderful effect upon the amount of light, and finally came the great discovery that it was a trace of ceria in admixture with the thoria that gave the mantle the marvellous power of emitting light.
Certain factors limit the number of oxides that can be used in the manufacture of an incandescent mantle. Atmospheric influences must not have any action upon them, and they must be sufficiently refractory not to melt or even soften to any extent at the temperature of the flame; they must also be non-volatile, whilst the shrinkage during the process of “burning off” must not be excessive. The following table gives the light-emissivity from pure and commercial samples of the oxides which most nearly conform to the above requirements; the effect of impurity upon the lighting power will be seen to be most marked.
| Pure. | Commercial. | |
| Metals— | ||
| Zirconia | 1.5 | 3.1 |
| Thoria | 0.5 | 6.0 |
| Earth metals— | ||
| Cerite earths—Ceria | 0.4 | 0.9 |
| Lanthania | 6.0 | |
| Yttrite earths—Yttria | 3.2 | |
| Erbia | 0.6 | 1.7 |
| Common earths—Chromium oxide | 0.4 | 0.4 |
| Alumina | 0.6 | 0.6 |
| Alkaline earth metals— | ||
| Baryta | 3.3 | 3.3 |
| Strontia | 5.2 | 5.5 |
| Magnesia | 5.0 | 5.0 |
Of these oxides thoria, when tested for shrinkage, duration and strength, stands pre-eminent. It is also possible to employ zirconia and alumina. Zirconia has the drawback that in the hottest part of the flame it is liable not only to shrinkage and semi-fusion, but also to slow volatilization, and the same objections hold good with respect to alumina. With thoria the shrinkage is smaller than with any other known substance, and it possesses very high refractory powers.
The factor which gives thoria its pre-eminence as the basis of the mantle is that in the conversion of thorium nitrate into thorium oxide by heat, an enormous expansion takes place, the oxide occupying more than ten times the volume of the nitrate. This means that the mass is highly spongy, and contains an enormous number of little air-cells which must render it an excellent non-conductor. A mantle made with thoria alone gives practically no light. But the power of light-emissivity is awakened by the addition of a small trace of ceria; and careful experiment shows that as ceria is added to it little by little, the light which the mantle emits grows greater and greater, until the ratio of 99% of thoria and 1% of ceria is reached, when the maximum illuminating effect is obtained. The further addition of ceria causes gradual diminution of light, until, when with some 10% of ceria has been added, the light given by the mantle is again almost inappreciable. When cerium nitrate is converted by heat into cerium oxide, the expansion which takes place is practically nil, the ceria obtained from a gramme of the nitrate occupying about the same space as the original nitrate. Thus, although by weight the ratio of ceria to thoria is as 1:99, by volume it is only as 1:999.
The most successful form of mantle is made by taking a cylinder of cotton net about 8 in. long, and soaking it in a solution of nitrates of the requisite metals until the microscopic fibres of the cotton are entirely filled Manufacture of mantles. with liquid. A longer soaking is not advantageous, as the acid nature of the liquid employed tends to weaken the fabric and render it more delicate to handle. The cotton is then wrung out to free it from the excess of liquid, and one end is sewn together with an asbestos thread, a loop of the same material or of thin platinum wire being fixed across the constricted portion to provide a support by which the mantle may be held by the carrying rod, which is either external to the mantle, or (as is most often the case) fixed centrally in the burner head. It is then ready for “burning off,” a process in which the organic matter is removed and the nitrates are converted into oxides. The flame of an atmospheric burner is first applied to the constricted portion at the top of the mantle, whereupon the cotton gradually burns downwards, the shape of the mantle to a great extent depending on the regularity with which the combustion takes place. A certain amount of carbon is left behind after the flame has died out, and this is burnt off by the judicious application of a flame from an atmospheric blast burner to the interior. The action which takes place during the burning off is as follows: The cellulose tubes of the fibre are filled with the crystallized nitrates of the metals used, and as the cellulose burns the nitrates decompose, giving up oxygen and forming fusible nitrites, which in their semi-liquid condition are rendered coherent by the rapid expansion as the oxide forms. As the action continues the nitrites become oxides, losing their fusibility, so that by the time the organic matter has disappeared a coherent thread of oxide is left in place of the nitrate-laden thread of cotton. In the early days of incandescent lighting the mantles had to be sent out unburnt, as no process was known by which the burnt mantle could be rendered sufficiently strong to bear carriage. As the success of a mantle depends upon its fitting the flame, and as the burning off requires considerable skill, this was a great difficulty. Moreover the acid nature of the nitrates in the fibres rapidly rotted them, unless they had been subjected to the action of ammonia gas, which neutralized any excess of acid. It was discovered, however, that the burnt-off mantle could be temporarily strengthened by dipping it in collodion, a solution of soluble gun-cotton in ether and alcohol together with a little castor-oil or similar material to prevent excessive shrinkage when drying. When the mantle was removed from the solution a thin film of solid collodion was left on it, and this could be burned away when required.
After the Welsbach mantle had proved itself a commercial success many attempts were made to evade the monopoly created under the patents, and, although it was found impossible to get the same illuminating power with anything but the mixture of 99% thoria and 1% ceria, many ingenious processes were devised which resulted in at least one improvement in mantle manufacture. One of the earliest attempts in this direction was the “Sunlight” mantle, in which cotton was saturated with the oxides of aluminium, chromium and zirconium, the composition of the burnt-off mantle being:—
| Alumina | 86.88 |
| Chromium oxide | 8.68 |
| Zirconia | 4.44 |
| ——— | |
| 100.00 |
The light given by these mantles was entirely dependent upon the proportion of chromium oxides present, the alumina playing the part of base in the same way that the thoria does in the Welsbach mantle, the zirconia being added merely to strengthen the structure. These mantles enjoyed considerable popularity owing to the yellowish pink light they emitted, but, although they could give an initial illumination of 12 to 15 candles per foot of gas consumed, they rapidly lost their light-giving power owing to the slow volatilization of the oxides of chromium and aluminium.
Another method of making the mantle was first to produce a basis of thoria, and, having got the fabric in thorium oxide, to coat it with a mixture of 99% thoria and 1% ceria. This modification seems to give an improvement in the initial amount of light given by the mantle. In the Voelker mantle a basis of thoria was produced, and was then coated by dipping in a substance termed by the patentee “Voelkerite,” a body made by fusing together a number of oxides in the electric furnace. The fused mass was then dissolved in the strongest nitric acid, and diluted with absolute alcohol to the necessary degree. A very good mantle having great lasting power was thus produced. It was claimed that the process of fusing the materials together in the electric furnace altered the composition in some unexplained way, but the true explanation is probably that all water of hydration was eliminated.
The “Daylight” mantle consisted of a basis of thoria or thoria mixed with zirconia, dipped in collodion containing a salt of cerium in solution; on burning off the collodion the ceria was left in a finely divided condition on the surface of the thoria. In this way a very high initial illuminating power was obtained, which, however, rapidly fell as the ceria slowly volatilized.
Perhaps the most interesting development of the Welsbach process was dependent upon the manufacture of filaments of soluble guncotton or collodion as in the production of artificial silk. In general the process consisted in forcing a thick solution of the nitrated cellulose through capillary glass tubes, the bore of which was less than the one-hundredth of a millimetre. Ten or twelve of the expressed fibres were then twisted together and wound on a bobbin, the air of the room being kept sufficiently heated to cause the drying of the filaments a few inches from the orifice of the tube. The compound thread was next denitrated to remove its extreme inflammability, and for this purpose the skeins were dipped in a solution of (for instance) ammonium sulphide, which converted them into ordinary cellulose. After washing and drying the skeins were ready for the weaving machines. In 1894 F. de Mare utilized collodion for the manufacture of a mantle, adding the necessary salts to the collodion before squeezing it into threads. O. Knöfler in 1895, and later on A. Plaissetty, took out patents for the manufacture of mantles by a similar process to De Mare’s, the difference between the two being that Knöfler used ammonium sulphide for the denitration of his fabric, whilst Plaissetty employed calcium sulphide, the objection to which is the trace of lime left in the material. Another method for making artificial silk which has a considerable reputation is that known as the Lehner process, which in its broad outlines somewhat resembles the Chardonnet, but differs from it in that the excessively high pressures used in the earlier method are done away with by using a solution of a more liquid character, the thread being hardened by passing through certain organic solutions. This form of silk lends itself perhaps better to the carrying of the salts forming the incandescent oxides than the previous solutions, and mantles made by this process, known as Lehner mantles, showed promise of being a most important development of De Mare’s original idea. Mantles made by these processes show that it is possible to obtain a very considerable increase in life and light-emissivity, but mantles made on this principle could not now be sold at a price which would enable them to compete with mantles of the Welsbach type.
The cause of the superiority of these mantles having been realized, developments in the required direction were made. The structure of the cotton mantle differed widely from that obtained by the various collodion processes, and this alteration in structure was mainly responsible for the increase in life. Whereas the average of a large number of Welsbach mantles tested only showed a useful life of 700 to 1000 hours, the collodion type would average about 1500 hours, some mantles being burnt for an even longer period and still giving an effective illumination. This being so, it was clear that one line of advance would be found in obtaining some material which, whilst giving a structure more nearly approaching that of the collodion mantle, would be sufficiently cheap to compete with the Welsbach mantle, and this was successfully done.
By the aid of the microscope the structure of the mantle can be clearly defined, and in examining the Welsbach mantle before and after burning, it will be noticed that the cotton thread is a closely twisted and plaited rope of myriads of minute fibres, whilst the collodion mantle is a bundle of separate filaments without plait or heavy twisting, the number of such filaments varying with the process by which it was made. This latter factor experiment showed to have a certain influence on the useful light-giving life of the mantle, as whereas the Knöfler and Plaissetty mantles had an average life of about 1500 hours, the Lehner fabric, which contained a larger number of finer threads, could often be burnt continuously for over 3000 hours, and at the end of that period gave a better light than most of the Welsbach after as many hundred.
It is well known that plaiting gave the cotton candle-wick that power of bending over, when freed from the binding effect of the candle material and influenced by heat, which brought the tip out from the side of the flame. This, by enabling the air to get at it and burn it away, removed the nuisance of having to snuff the candle, which for many centuries has rendered it a tiresome method of lighting. In the cotton mantle, the tight twisting of the fibre brings this torsion into play. When the cotton fibres saturated with the nitrates of the rare metals are burnt off, and the conversion into oxides takes place, as the cotton begins to burn, not only does the shrinkage of the mass throw a strain on the oxide skeleton, but the last struggle of torsion in the burning of the fibre tends towards disintegration of the fragile mass, and this all plays a part in making the cotton mantle inferior to the collodion type.
If ramie fibre be prepared in such a way as to remove from it all traces of the glutinous coating, a silk-like fabric can be obtained from it, and if still further prepared so as to improve its absorbent powers, it can be formed into mantles having a life considerably greater than is possessed by those of the cotton fabric. Ramie thus seemed likely to yield a cheap competitor in length of endurance to the collodion mantle, and results have justified this expectation. By treating the fibre so as to remove the objections against its use for mantle-making, and then making it into threads with the least possible amount of twist, a mantle fabric can be made in every way superior to that given by cotton.
The Plaissetty mantles, which as now manufactured also show a considerable advance in life and light over the original Welsbach mantles, are made by impregnating stockings of either cotton or ramie with the nitrates of thorium and cerium in the usual way, and, before burning off, mercerizing the mantle by steeping in ammonia solution, which converts the nitrates into hydrates, and gives greater density and strength to the finished mantle. The manufacturers of the Plaissetty mantle have also made a modification in the process by which the saturated fabric can be so prepared as to be easily burnt off by the consumer on the burner on which it is to be used, in this way doing away with the initial cost of burning off, shaping, hardening and collodionizing.
Since 1897 inventions have been patented for methods of intensifying the light produced by burning gas under a mantle and increasing the light generated per unit volume of gas. The systems have either been self-intensifying Intensifying systems. or have depended on supplying the gas (or gas and air) under an increased pressure. Of the self-intensifying systems those of Lucas and Scott-Snell have been the most successful. A careful study has been made by the inventor of the Lucas light of the influence of various sizes and shapes of chimneys in the production of draught. The specially formed chimney used exerts a suction on the gas flame and air, and the burner and mantle are so constructed as to take full advantage of the increased air supply, with the result that the candle power given by the mantle is considerably augmented. With the Scott-Snell system the results obtained are about the same as those given by the Lucas light, but in this case the waste heat from the burner is caused to operate a plunger working in the crown of the lamp which sucks and delivers gas to the burner. Both these systems are widely used for public lighting in many large towns of the United Kingdom and the continent of Europe.
The other method of obtaining high light-power from incandescent gas burners necessitates the use of some form of motive power in order to place the gas, or both gas and air, under an increased pressure. The gas compressor is worked by a water motor, hot air or gas engine; a low pressure water motor may be efficiently driven by water from the main, but with large installations it is more economical to drive the compressor by a gas engine. To overcome the intermittent flow of gas caused by the stroke of the engine, a regulator on the floating bell principle is placed after the compressor; the pressure of gas in the apparatus governs automatically the flow of gas to the engine. With the Sugg apparatus for high power lighting the gas is brought from the district pressure, which is equal to about 2½ in. of water, to an average of 12 in. water pressure. The light obtained by this system when the gas pressure is 9½ in. is 300 candle power with an hourly consumption of 10 cub. ft. of gas, equivalent to 30 candles per cubic foot, and with a gas pressure equal to 14 in. of water 400 candles are obtained with an hourly consumption of 12½ cub. ft., which represents a duty of 32 candles per cubic foot of gas consumed. High pressure incandescent lighting makes it possible to burn a far larger volume of gas in a given time under a mantle than is the case with low pressure lighting, so as to create centres of high total illuminating value to compete with arc lighting in the illumination of large spaces, and the Lucas, Keith, Scott-Snell, Millennium, Selas, and many other pressure systems answer most admirably for this purpose.
The light given by the ordinary incandescent mantle burning in an upright position tends rather to the upward direction, because owing to the slightly conical shape of the mantle the maximum light is emitted at an angle a Inverted burners. little above the horizontal. Inasmuch as for working purposes the surface that a mantle illuminates is at angles below 45° from the horizontal, it is evident that a considerable loss of efficient lighting is brought about, whilst directly under the light the burner and fittings throw a strong shadow. To avoid this trouble attempts have from time to time been made to produce inverted burners which should heat a mantle suspended below the mouth of the burner. As early as 1882 Clamond made what was practically an inverted gas and air blowpipe to use with his incandescent basket, but it was not until 1900-1901 that the inverted mantle became a possibility. Although there was a strong prejudice against it at first, as soon as a really satisfactory burner was introduced, its success was quickly placed beyond doubt. The inverted mantle has now proved itself one of the chief factors in the enormous success achieved by incandescent mantle lighting, as the illumination given by it is far more efficient than with the upright mantle, and it also lends itself well to ornamental treatment.
When the incandescent mantle was first introduced in 1886 an ordinary laboratory Bunsen burner was experimentally employed, but unless a very narrow mantle just fitting the top of the tube was used the flame could Burners. not be got to fit the mantle, and it was only the extreme outer edge of the flame which endowed the mantle fabric with the high incandescent. A wide burner top was then placed on the Bunsen tube so as to spread the flame, and a larger mantle became possible, but it was then found that the slowing down of the rate of flow at the mouth of the burner owing to its enlargement caused flashing or firing back, and to prevent this a wire gauze covering was fitted to the burner head; and in this way the 1886-1887 commercial Welsbach burner was produced. The length of the Bunsen tube, however, made an unsightly fitting, so it was shortened, and the burner head made to slip over it, whilst an external lighting back plate was added. The form of the “C” burner thus arrived at has undergone no important further change. When later on it was desired to make incandescent mantle burners that should not need the aid of a chimney to increase the air supply, the long Bunsen tube was reverted to, and the Kern, Bandsept, and other burners of this class all have a greater total length than the ordinary burners. To secure proper mixing of the air and gas, and to prevent flashing back, they all have heads fitted with baffles, perforations, gauze, and other devices which oppose considerable resistance to the flow of the stream of air and gas.
In 1900, therefore, two classes of burner were in commercial existence for incandescent lighting—(1) the short burner with chimney, and (2) the long burner without chimney. Both classes had the burner mouth closed with gauze or similar device, and both needed as an essential that the mantle should fit closely to the burner head.
Prior to 1900 attempts had been made to construct a burner in which an incandescent mantle should be suspended head downwards. Inventors all turned to the overhead regenerative gas lamps of the Wenham type, or the inverted blowpipe used by Clamond, and in attempting to make an inverted Bunsen employed either artificial pressure to the gas or the air, or to both, or else enclosed the burner and mantle in a globe, and by means of a long chimney created a strong draught. These burners also were all regenerative and aimed at heating the air or gas or mixture of the two, and they had the further drawback of being complicated and costly. Regeneration is a valuable adjunct in ordinary gas lighting as it increases the actions that liberate the carbon particles upon which the luminosity of a flame is dependent, and also increases the temperature; but with the mixture of air and gas in a Bunsen regeneration is not a great gain when low and is a drawback when intense, because incipient combination is induced between the oxygen of the air and the coal-gas before the burner head is reached, the proportions of air and gas are disturbed, and the flame instead of being non-luminous shows slight luminosity and tends to blacken the mantle. The only early attempt to burn a mantle in an inverted position without regeneration or artificial pressure or draught was made by H. A. Kent in 1897, and he used, not an inverted Bunsen, but one with the top elongated and turned over to form a siphon, so that the point of admixture of air and gas was below the level of the burner head, and was therefore kept cool and away from the products of combustion.
In 1900 J. Bernt and E. Cérvenka set themselves to solve the problem of making a Bunsen burner which should consume gas under ordinary gas pressure in an inverted mantle. They took the short Bunsen burner, as found in the most commonly used upright incandescent burners, and fitted to it a long tube, preferably of non-conducting material, which they called an isolator, and which is designed to keep the flame at a distance from the Bunsen. They found that it burnt fairly well, and that the tendency of the flame to burn or lap back was lessened, but that the hot up-current of heated air and products of combustion streamed up to the air holes of the Bunsen, and by contaminating the air supply caused the flame to pulsate. They then fixed an inverted cone on the isolator to throw the products of combustion outwards and away from the air holes, and found that the addition of this “deflecting cone” steadied the flame. Having obtained a satisfactory flame, they attacked the problem of the burner head. Experiments showed that the burner head must be not only open but also of the same size or smaller than the burner tube, and that by projecting it downwards into the mantle and leaving a space between the mantle and the burner head the maximum mantle surface heated to incandescence was obtained. It was also found that the distance which the burner head projects into the mantle is equivalent to the same amount of extra water pressure on the gas, and with a long mantle it was found useful under certain conditions to add a cylinder or sleeve with perforated sides to carry the gas still lower into the mantle. The principles thus set forth by Kent, Bernt and Cérvenka form the basis of construction of all the types of inverted mantle burners which so greatly increased the popularity of incandescent gas lighting at the beginning of the 20th century, whilst improvements in the shape of the mantle for inverted lighting and the methods of attachment to the burner have added to the success achieved.
The wonderful increase in the amount of light that can be obtained from gas by the aid of the incandescent gas mantle is realized when one compares the 1 to 3.2 candles per cubic foot given by the burners used in the middle of the 19th century with the duty of incandescent burners, as shown in the following table:—
Light yielded per cubic foot of Gas.
| Burner. | Candle power. |
| Low pressure upright incandescent burners | 15 to 20 candles |
| Inverted burners | 14 to 21 ” |
| Kern burners | 20 to 24 ” |
| High pressure burners | 22 to 36 ” |
3. Electric Lighting.
Electric lamps are of two varieties: (1) Arc Lamps and (2) Incandescent or Glow Lamps. Under these headings we may briefly consider the history, physical principles, and present practice of the art of electric lighting.
1. Arc Lamps.—If a voltaic battery of a large number of cells has its terminal wires provided with rods of electrically-conducting carbon, and these are brought in contact and then slightly separated, a form of electric discharge takes place between them called the electric arc. It is not quite certain who first observed this effect of the electric current. The statement that Sir Humphry Davy, in 1801, first produced and studied the phenomenon is probably correct. In 1808 Davy had provided for him at the Royal Institution a battery of 2000 cells, with which he exhibited the electric arc on a large scale.
The electric arc may be produced between any conducting materials maintained at different potentials, provided that the source of electric supply is able to furnish a sufficiently large current; but for illuminating purposes pieces of hard graphitic carbon are most convenient. If some source of continuous electric current is connected to rods of such carbon, first brought into contact and then slightly separated, the following facts may be noticed: With a low electromotive force of about 50 or 60 volts no discharge takes place until the carbons are in actual contact, unless the insulation of the air is broken down by the passage of a small electric spark. When this occurs, the space between the carbons is filled at once with a flame or luminous vapour, and the carbons themselves become highly incandescent at their extremities. If they are horizontal the flame takes the form of an arch springing between their tips; hence the name arc. This varies somewhat in appearance according to the nature of the current, whether continuous or alternating, and according as it is formed in the open air or in an enclosed space to which free access of oxygen is prevented. Electric arcs between metal surfaces differ greatly in colour according to the nature of the metal. When formed by an alternating current of high electromotive force they resemble a lambent flame, flickering and producing a somewhat shrill humming sound.
Electric arcs may be classified into continuous or alternating current arcs, and open or enclosed arcs, carbon arcs with pure or chemically impregnated carbons, or so-called flame arcs, and arcs formed with metallic or oxide electrodes, such as magnetite. A continuous current arc is formed with an electric current flowing always in the same direction; an alternating current arc is formed with a periodically reversed current. An open arc is one in which the carbons or other material forming the arc are freely exposed to the air; an enclosed arc is one in which they are included in a glass vessel. If carbons impregnated with various salts are used to colour or increase the light, the arc is called a chemical or flame arc. The carbons or electrodes may be arranged in line one above the other, or they may be inclined so as to project the light downwards or more in one direction. In a carbon arc if the current is continuous the positive carbon becomes much hotter at the end than the negative, and in the open air it is worn away, partly by combustion, becoming hollowed out at the extremity into a crater. At the same time the negative carbon gradually becomes pointed, and also wears away, though much less quickly than the positive. In the continuous-current open arc the greater part of the light proceeds from the highly incandescent positive crater. When the arc is examined through dark glasses, or by the optical projection of its image upon a screen, a violet band or stream of vapour is seen to extend between the two carbons, surrounded by a nebulous golden flame or aureole. If the carbons are maintained at the right distance apart the arc remains steady and silent, but if the carbons are impure, or the distance between them too great, the true electric arc rapidly changes its place, flickering about and frequently becoming extinguished; when this happens it can only be restored by bringing the carbons once more into contact. If the current is alternating, then the arc is symmetrical, and both carbons possess nearly the same appearance. If it is enclosed in a vessel nearly air-tight, the rate at which the carbons are burnt away is greatly reduced, and if the current is continuous the positive carbon is no longer cratered out and the negative no longer so much pointed as in the case of the open arc.
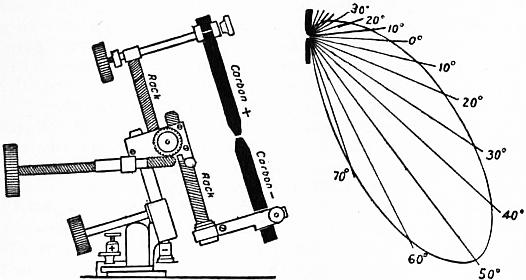
Davy used for his first experiments rods of wood charcoal which had been heated and plunged into mercury to make them better conductors. Not until 1843 was it proposed by J. B. L. Foucault to employ pencils Carbons. cut from the hard graphitic carbon deposited in the interior of gas retorts. In 1846 W. Greener and W. E. Staite patented a process for manufacturing carbons for this purpose, but only after the invention of the Gramme dynamo in 1870 any great demand arose for them. F. P. É. Carré in France in 1876 began to manufacture arc lamp carbons of high quality from coke, lampblack and syrup. Now they are made by taking some specially refined form of finely divided carbon, such as the soot or lampblack formed by cooling the smoke of burning paraffin or tar, or by the carbonization of organic matter, and making it into a paste with gum or syrup. This carbon paste is forced through dies by means of a hydraulic press, the rods thus formed being subsequently baked with such precautions as to preserve them perfectly straight. In some cases they are cored, that is to say, have a longitudinal hole down them, filled in with a softer carbon. Sometimes they are covered with a thin layer of copper by electro-deposition. They are supplied for the market in sizes varying from 4 or 5 to 30 or 40 millimetres in diameter, and from 8 to 16 in. in length. The value of carbons for arc lighting greatly depends on their purity and freedom from ash in burning, and on perfect uniformity of structure. For ordinary purposes they are generally round in section, but for certain special uses, such as lighthouse work, they are made fluted or with a star-shaped section. The positive carbon is usually of larger section than the negative. For continuous-current arcs a cored carbon is generally used as a positive, and a smaller solid carbon as a negative. For flame arc lamps the carbons are specially prepared by impregnating them with salts of calcium, magnesium and sodium. The calcium gives the best results. The rod is usually of a composite type. The outer zone is pure carbon to give strength, the next zone contains carbon mixed with the metallic salts, and the inner core is the same but less compressed. In addition to the metallic salts a flux has to be introduced to prevent the formation of a non-conducting ash, and this renders it desirable to place the carbons in a downward pointing direction to get rid of the slag so formed. Bremer first suggested in 1898 for this purpose the fluorides of calcium, strontium or barium. When such carbons are used to form an electric arc the metallic salts deflagrate and produce a flame round the arc which is strongly coloured, the object being to produce a warm yellow glow, instead of the somewhat violet and cold light of the pure carbon arc, as well as a greater emission of light. As noxious vapours are however given off, flame arcs can only be used out of doors. Countless researches have been made on the subject of carbon manufacture, and the art has been brought to great perfection.
Special manuals must be consulted for further information (see especially a treatise on Carbon making for all electrical purposes, by F. Jehl, London, 1906).
 |
|
| Fig. 4. | Fig. 5. |
The physical phenomena of the electric arc are best examined by forming a carbon arc between two carbon rods of the above description, held in line in a special apparatus, and arranged so as to be capable of being moved to or from Physical phenomena. each other with a slow and easily regulated motion. An arrangement of this kind is called a hand-regulated arc lamp (fig. 4). If such an arc lamp is connected to a source of electric supply having an electromotive force preferably of 100 volts, and if some resistance is included in the circuit, say about 5 ohms, a steady and continuous arc is formed when the carbons are brought together and then slightly separated. Its appearance may be most conveniently examined by projecting its image upon a screen of white paper by means of an achromatic lens. A very little examination of the distribution of light from the arc shows that the illuminating or candle-power is not the same in different directions. If the carbons are vertical and the positive carbon is the upper of the two, the illuminating power is greatest in a direction at an angle inclined about 40 or 50 degrees below the horizon, and at other directions has different values, which may be represented by the lengths of radial lines drawn from a centre, the extremities of which define a curve called the illuminating curve of the arc lamp (fig. 5). Considerable differences exist between the forms of the illuminating-power curves of the continuous and alternating current and the open or enclosed arcs. The chief portion of the emitted light proceeds from the incandescent crater; hence the form of the illuminating-power curve, as shown by A. P. Trotter in 1892, is due to the apparent area of the crater surface which is visible to an eye regarding the arc in that direction. The form of the illuminating-power curve varies with the length of the arc and relative size of the carbons. Leaving out of account for the moment the properties of the arc as an illuminating agent, the variable factors with which we are concerned are (i.) the current through the arc; (ii.) the potential difference of the carbons; (iii.) the length of the arc; and (iv.) the size of the carbons. Taking in the first place the typical direct-current arc between solid carbons, and forming arcs of different lengths and with carbons of different sizes, it will be found that, beginning at the lowest current capable of forming a true arc, the potential difference of the carbons (the arc P.D.) decreases as the current increases. Up to a certain current strength the arc is silent, but at a particular critical value P.D. suddenly drops about 10 volts, the current at the same time rising 2 or 3 amperes. At that moment the arc begins to hiss, and in this hissing condition, if the current is still further increased, P.D. remains constant over wide limits. This drop in voltage on hissing was first noticed by A. Niaudet (La Lumière électrique, 1881, 3, p. 287). It has been shown by Mrs Ayrton (Journ. Inst. Elec. Eng. 28, 1899, p. 400) that the hissing is mainly due to the oxygen which gains access from the air to the crater, when the latter becomes so large by reason of the increase of the current as to overspread the end of the positive carbon. According to A. E. Blondel and Hans Luggin, hissing takes place whenever the current density becomes greater than about 0.3 or 0.5 ampere per square millimetre of crater area.
The relation between the current, the carbon P.D., and the length of arc in the case of the direct-current arc has been investigated by many observers with the object of giving it mathematical expression.
Let V stand for the potential difference of the carbons in volts, A for the current through the arc in amperes, L for the length of the arc in millimetres, R for the resistance of the arc; and let a, b, c, d, &c., be constants. Erik Edlund in 1867, and other workers after him, considered that their experiments showed that the relation between V and L could be expressed by a simple linear equation,
V = a + bL.
Later researches by Mrs Ayrton (Electrician, 1898, 41, p. 720), however, showed that for a direct-current arc of given size with solid carbons, the observed values of V can be better represented as a function both of A and of L of the form
| V = a + bL + | c + dL | . |
| A |
In the case of direct-current arcs formed with solid carbons, Edlund and other observers agree that the arc resistance R may be expressed by a simple straight line law, R = e + fL. If the arc is formed with cored carbons, Mrs Ayrton demonstrated that the lines expressing resistance as a function of arc length are no longer straight, but that there is a rather sudden dip down when the length of the arc is less than 3 mm.
The constants in the above equation for the potential difference of the carbons were determined by Mrs Ayrton in the case of solid carbons to be—
| V = 38.9 + 2.07L + | 11.7 + 10.5L | . |
| A |
There has been much debate as to the meaning to be given to the constant a in the above equation, which has a value apparently not far from forty volts for a direct-current arc with solid carbons. The suggestion made in 1867 by Edlund (Phil. Mag., 1868, 36, p. 358), that it implied the existence of a counter-electromotive force in the arc, was opposed by Luggin in 1889 (Wien. Ber. 98, p. 1198), Ernst Lecher in 1888 (Wied. Ann., 1888, 33, p. 609), and by Franz Stenger in 1892 (Id. 45, p. 33); whereas Victor von Lang and L. M. Arons in 1896 (Id. 30, p. 95), concluded that experiment indicated the presence of a counter-electromotive force of 20 volts. A. E. Blondel concludes, from experiments made by him in 1897 (The Electrician, 1897, 39, p. 615), that there is no counter-electromotive force in the arc greater than a fraction of a volt. Subsequently W. Duddëll (Proc. Roy. Soc., 1901, 68, p. 512) described experiments tending to prove the real existence of a counter-electromotive force in the arc, probably having a thermo-electric origin, residing near the positive electrode, and of an associated lesser adjuvant e.m.f. near the negative carbon.
This fall in voltage between the carbons and the arc is not uniformly distributed. In 1898 Mrs Ayrton described the results of experiments showing that if V1 is the potential difference between the positive carbon and the arc, then
| V1 = 31.28 + | 9 + 3.1L | ; |
| A |
and if V2 is the potential difference between the arc and the negative carbon, then
| V2 = 7.6 + | 13.6 | , |
| A |
where A is the current through the arc in amperes and L is the length of the arc in millimetres.
The total potential difference between the carbons, minus the fall in potential down the arc, is therefore equal to the sum of V1 + V2 = V3.
| Hence V3 = 38.88 + | 22.6 + 3.1L | . |
| A |
The difference between this value and the value of V, the total potential difference between the carbons, gives the loss in potential due to the true arc. These laws are simple consequences of straight-line laws connecting the work spent in the arc at the two electrodes with the other quantities. If W be the work spent in the arc on either carbon, measured by the product of the current and the potential drop in passing from the carbon to the arc, or vice versa, then for the positive carbon W = a + bA, if the length of arc is constant, W = c + dL, if the current through the arc is constant, and for the negative carbon W = e + fA.
In the above experiments the potential difference between the carbons and the arc was measured by using a third exploring carbon as an electrode immersed in the arc. This method, adopted by Lecher, F. Uppenborn, S. P. Thompson, and J. A. Fleming, is open to the objection that the introduction of the third carbon may to a considerable extent disturb the distribution of potential.
The total work spent in the continuous-current arc with solid carbons may, according to Mrs Ayrton, be expressed by the equation
W = 11.7 + 10.5L + (38.9 + 2.07L) A.
It will thus be seen that the arc, considered as a conductor, has the property that if the current through it is increased, the difference of potential between the carbons is decreased, and in one sense, therefore, the arc may be said to act as if it were a negative resistance. Frith and Rodgers (Electrician, 1896, 38, p. 75) have suggested that the resistance of the arc should be measured by the ratio between a small increment of carbon potential difference and the resulting small increment of current; in other words, by the equation dV/dA, and not by the ratio simply of V:A. Considerable discussion has taken place whether an electrical resistance can have a negative value, belonging as it does to the class of scalar mathematical quantities. Simply considered as an electrical conductor, the arc resembles an intensely heated rod of magnesia or other refractory oxide, the true resistance of which is decreased by rise of temperature. Hence an increase of current through such a rod of refractory oxide is accompanied by a decrease in the potential difference of the ends. This, however, does not imply a negative resistance, but merely the presence of a resistance with a negative temperature coefficient. If we plot a curve such that the ordinates are the difference of potential of the carbons and the abscissae the current through the arc for constant length of arc, this curve is now called a characteristic curve of the arc and its slope at any point the instantaneous resistance of the arc.
Other physical investigations have been concerned with the intrinsic brightness of the crater. It has been asserted by many observers, such as Blondel, Sir W. de W. Abney, S. P. Thompson, Trotter, L. J. G. Violle and others, that this is practically independent of the current passing, but great differences of opinion exist as to its value. Abney’s values lie between 39 and 116, Trotter’s between 80 and 170 candles per square millimetre. Blondel in 1893 made careful determinations of the brightness of the arc crater, and came to the conclusion that it was 160 candles per square millimetre. Subsequently J. E. Petavel found a value of 147 candles per square millimetre for current densities varying from .06 to .26 amperes per square millimetre (Proc. Roy. Soc., 1899, 65, p. 469). Violle also, in 1893, supported the opinion that the brightness of the crater per square millimetre was independent of the current density, and from certain experiments and assumptions as to the specific heat of carbon, he asserted the temperature of the crater was about 3500° C. It has been concluded that this constancy of temperature, and therefore of brightness, is due to the fact that the crater is at the temperature of the boiling-point of carbon, and in that case its temperature should be raised by increasing the pressure under which the arc works. W. E. Wilson in 1895 attempted to measure the brightness of the crater under various pressures, and found that under five atmospheres the resistance of the arc appeared to increase and the temperature of the crater to fall, until at a pressure of 20 atmospheres the brightness of the crater had fallen to a dull red. In a later paper Wilson and G. F. Fitzgerald stated that these preliminary experiments were not confirmed, and their later researches throw considerable doubt on the suggestion that it is the boiling-point of carbon which determines the temperature of the crater. (See Electrician, 1895, 35, p. 260, and 1897, 38, p. 343.)
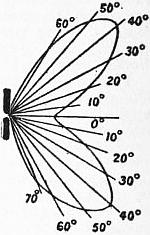 |
| Fig. 6. |
The study of the alternating-current arc has suggested a number of new experimental problems for investigators. In this case all the factors, namely, current, carbon P.D., resistance, and illuminating power, are periodically Alternating current arc. varying; and as the electromotive force reverses itself periodically, at certain instants the current through the arc is zero. As the current can be interrupted for a moment without extinguishing the arc, it is possible to work the electric arc from an alternating current generator without apparent intermission in the light, provided that the frequency is not much below 50. During the moment that the current is zero the carbon continues to glow. Each carbon in turn becomes, so to speak, the crater carbon, and the illuminating power is therefore symmetrically distributed. The curve of illumination is as shown in fig. 3. The nature of the variation of the current and arc P.D. can be examined by one of two methods, or their modifications, originally due to Jules Joubert and A. E. Blondel. Joubert’s method, which has been perfected by many observers, consists in attaching to the shaft of the alternator a contact which closes a circuit at an assigned instant during the phase. This contact is made to complete connexion either with a voltmeter or with a galvanometer placed as a shunt across the carbons or in series with the arc. By this arrangement these instruments do not read, as usual, the root-mean-square value of the arc P.D. or current, but give a constant indication determined by, and indicating, the instantaneous values of these quantities at some assigned instant. By progressive variation of the phase-instant at which the contact is made, the successive instantaneous values of the electric quantities can be measured and plotted out in the form of curves. This method has been much employed by Blondel, Fleming, C. P. Steinmetz, Tobey and Walbridge, Frith, H. Görges and many others. The second method, due to Blondel, depends on the use of the Oscillograph, which is a galvanometer having a needle or coil of very small periodic time of vibration, say 1⁄2000th part of a second or less, so that its deflections can follow the variations of current passing through the galvanometer. An improved form of oscillograph, devised by Duddell, consists of two fine wires, which are strained transversely to the lines of flux of a strong magnetic field (see Oscillograph). The current to be examined is made to pass up one wire and down the other, and these wires are then slightly displaced in opposite directions. A small mirror attached to the wires is thus deflected rapidly to and fro in synchronism with the variations of the current. From the mirror a ray of light is reflected which falls upon a photographic plate made to move across the field with a uniform motion. In this manner a photographic trace can be obtained of the wave form. By this method the variations of electric quantities in an alternating-current arc can be watched. The variation of illuminating power can be followed by examining and measuring the light of the arc through slits in a revolving stroboscopic disk, which is driven by a motor synchronously with the variation of current through the arc.
The general phenomena of the alternating-current arc are as follow:—
If the arc is supplied by an alternator of low inductance, and soft or cored carbons are employed to produce a steady and silent arc, the potential difference of the carbons periodically varies in a manner not very different from that of the alternator on open circuit. If, however, hard carbons are used, the alternating-current arc deforms the shape of the alternator electromotive force curve; the carbon P.D. curve may then have a very different form, and becomes, in general, more rectangular in shape, usually having a high peak at the front. The arc also impresses the deformation on the current curve. Blondel in 1893 (Electrician, 32, p. 161) gave a number of potential and current curves for alternating-current arcs, obtained by the Joubert contact method, using two movable coil galvanometers of high resistance to measure respectively potential difference and current. Blondel’s deductions were that the shape of the current and volt curves is greatly affected by the nature of the carbons, and also by the amount of inductance and resistance in the circuit of the alternator. Blondel, W. E. Ayrton, W. E. Sumpner and Steinmetz have all observed that the alternating-current arc, when hissing or when formed with uncored carbons, acts like an inductive resistance, and that there is a lag between the current curves and the potential difference curves. Hence the power-factor, or ratio between the true power and the product of the root-mean-square values of arc current and carbon potential difference, in this case is less than unity. For silent arcs Blondel found power-factors lying between 0.88 and 0.95, and for hissing ones, values such as 0.70. Ayrton and Sumpner stated that the power-factor may be as low as 0.5. Joubert, as far back as 1881, noticed the deformation which the alternating-current arc impresses upon the electromotive force curve of an alternator, giving an open circuit a simple harmonic variation of electromotive force. Tobey and Walbridge in 1890 gave the results of a number of observations taken with commercial forms of alternating-current arc lamps, in which the same deformation was apparent. Blondel in 1896 came to the conclusion that with the same alternator we can produce carbon P.D. curves of very varied character, according to the material of the core, the length of the arc, and the inductance of the circuit. Hard carbons gave a P.D. curve with a flat top even when worked on a low inductance alternator.
The periodic variation of light in the alternating-current arc has also been the subject of inquiry. H. Görges in 1895 at Berlin applied a stroboscopic method to steady the variations of illuminating power. Fleming and Petavel employed a similar arrangement, driving the stroboscopic disk by a synchronous motor (Phil. Mag., 1896, 41). The light passing through slits of the disk was selected in one particular period of the phase, and by means of a lens could be taken from any desired portion of the arc or the incandescent carbons. The light so selected was measured relatively to the mean value of the horizontal light emitted by the arc, and accidental variations were thus eliminated. They found that the light from any part is periodic, but owing to the slow cooling of the carbons never quite zero, the minimum value happening a little later than the zero value of the current. The light emitted by a particular carbon when it is the negative, does not reach such a large maximum value as when it is the positive. The same observers made experiments which seemed to show that for a given expenditure of power in the arc the alternating current arc in general gives less mean spherical candle-power than the continuous current one.
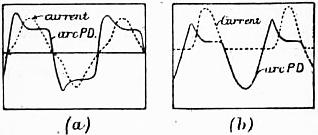 |
| Fig. 7. |
The effect of the wave form on the efficiency of the alternating-current arc has engaged the attention of many workers. Rössler and Wedding in 1894 gave an account of experiments with alternating-current arcs produced by alternators having electromotive force curves of very different wave forms, and they stated that the efficiency or mean spherical candle-power per watt expended in the arc was greatest for the flattest of the three wave forms by nearly 50%. Burnie in 1897 gave the results of experiments of the same kind. His conclusion was, that since the light of the arc is a function of the temperature, that wave form of current is most efficient which maintains the temperature most uniformly throughout the half period. Hence, generally, if the current rises to a high value soon after its commencement, and is preserved at that value, or nearly at that value, during the phase, the efficiency of the arc will be greater when the current curve is more pointed or peaked. An important contribution to our knowledge concerning alternating-current arc phenomena was made in 1899 by W. Duddell and E. W. Marchant, in a paper containing valuable results obtained with their improved oscillograph.1 They studied the behaviour of the alternating-current arc when formed both with solid carbons, with cored carbons, and with carbon and metal rods. They found that with solid carbons the arc P.D. curve is always square-shouldered and begins with a peak, as shown in fig. 7 (a), but with cored carbons it is more sinusoidal. Its shape depends on the total resistance in the circuit, but is almost independent of the type of alternator, whereas the current wave form is largely dependent on the machine used, and on the nature and amount of the impedance in the circuit; hence the importance of selecting a suitable alternator for operating alternating-current arcs. The same observers drew attention to the remarkable fact that if the arc is formed between a carbon and metal rod, say a zinc rod, there is a complete interruption of the current over half a period corresponding to that time during which the carbon is positive; this suggests that the rapid cooling of the metal facilitates the flow of the current from it, and resists the flow of current to it. The dotted curve in fig. 7 (b) shows the current curve form in the case of a copper rod. By the use of the oscillograph Duddell and Marchant showed that the hissing continuous-current arc is intermittent, and that the current is oscillatory and may have a frequency of 1000 per second. They also showed that enclosing the arc increases the arc reaction, the front peak of the potential curve becoming more marked and the power-factor of the arc reduced.
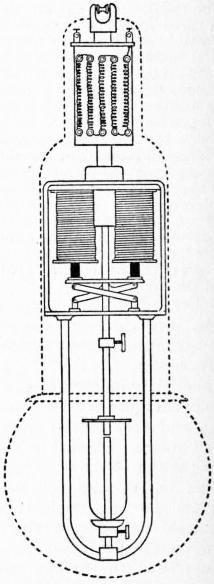 |
| Fig. 8.—Enclosed Arc Lamp. |
If a continuous-current electric arc is formed in the open air with a positive carbon having a diameter of about 15 millimetres, and a negative carbon having a diameter of about 9 millimetres, and if a current of 10 amperes is employed, Enclosed arc lamps. the potential difference between the carbons is generally from 40 to 50 volts. Such a lamp is therefore called a 500-watt arc. Under these conditions the carbons each burn away at the rate of about 1 in. per hour, actual combustion taking place in the air which gains access to the highly-heated crater and negative tip; hence the most obvious means of preventing this disappearance is to enclose the arc in an air-tight glass vessel. Such a device was tried very early in the history of arc lighting. The result of using a completely air-tight globe, however, is that the contained oxygen is removed by combustion with the carbon, and carbon vapour or hydrocarbon compounds diffuse through the enclosed space and deposit themselves on the cool sides of the glass, which is thereby obscured. It was, however, shown by L. B. Marks (Electrician 31, p. 502, and 38, p. 646) in 1893, that if the arc is an arc formed with a small current and relatively high voltage, namely, 80 to 85 volts, it is possible to admit air in such small amount that though the rate of combustion of the carbons is reduced, yet the air destroys by oxidation the carbon vapour escaping from the arc. An arc lamp operated in this way is called an enclosed arc lamp (fig. 8). The top of the enclosing bulb is closed by a gas check plug which admits through a small hole a limited supply of air. The peculiarity of an enclosed arc lamp operated with a continuous current is that the carbons do not burn to a crater on the positive, and a sharp tip or mushroom on the negative, but preserve nearly flat surfaces. This feature affects the distribution of the light. The illuminating curve of the enclosed arc, therefore, has not such a strongly marked maximum value as that of the open arc, but on the other hand the true arc or column of incandescent carbon vapour is less steady in position, wandering round from place to place on the surface of the carbons. As a compensation for this defect, the combustion of the carbons per hour in commercial forms of enclosed arc lamps is about one-twentieth part of that of an open arc lamp taking the same current.
It was shown by Fleming in 1890 that the column of incandescent carbon vapour constituting the true arc possesses a unilateral conductivity (Proc. Roy. Inst. 13, p. 47). If a third carbon is dipped into the arc so as to constitute a third pole, and if a small voltaic battery of a few cells, with a galvanometer in circuit, is connected in between the middle pole and the negative carbon, it is found that when the negative pole of the battery is in connexion with the negative carbon the galvanometer indicates a current, but does not when the positive pole of the battery is in connexion with the negative carbon of the arc.
Turning next to the consideration of the electric arc as a source of light, we have already noticed that the illuminating power in different directions is not the same. If we imagine an electric arc, formed between a pair of The arc as an illuminant. vertical carbons, to be placed in the centre of a hollow sphere painted white on the interior, then it would be found that the various zones of this sphere are unequally illuminated. If the points in which the carbons when prolonged would intercept the sphere are called the poles, and the line where the horizontal plane through the arc would intercept the sphere is called the equator, we might consider the sphere divided up by lines of latitude into zones, each of which would be differently illuminated. The total quantity of light or the total illumination of each zone is the product of the area of the zone and the intensity of the light falling on the zone measured in candle-power. We might regard the sphere as uniformly illuminated with an intensity of light such that the product of this intensity and the total surface of the sphere was numerically equal to the surface integral obtained by summing up the products of the areas of all the elementary zones and the intensity of the light falling on each. This mean intensity is called the mean spherical candle-power of the arc. If the distribution of the illuminating power is known and given by an illumination curve, the mean spherical candle-power can be at once deduced (La Lumière électrique, 1890, 37, p. 415).
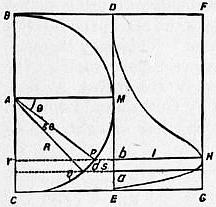 |
| Fig. 9. |
Let BMC (fig. 9) be a semicircle which by revolution round the diameter BC sweeps out a sphere. Let an arc be situated at A, and let the element of the circumference PQ = ds sweep out a zone of the sphere. Let the intensity of light falling on this zone be I. Then if θ ≈ the angle MAP and dθ the incremental angle PAQ, and if R is the radius of the sphere, we have
ds = Rdθ;
also, if we project the element PQ on the line DE we have
| ab = | ds cos θ, |
| ∴ ab = | R cos θdθ |
| and Iab = | IR cos θdθ. |
Let r denote the radius PT of the zone of the sphere, then
r = R cos θ.
Hence the area of the zone swept out by PQ is equal to
2πR cos θ ds = 2πR2 cos θdθ
in the limit, and the total quantity of light falling on the zone is equal to the product of the mean intensity or candle-power I in the direction AP and the area of the zone, and therefore to
2πIR2 cos θdθ.
Let I0 stand for the mean spherical candle-power, that is, let I0 be defined by the equation
4πR2I0 = 2πRΣ(Iab)
where Σ(Iab) is the sum of all the light actually falling on the sphere surface, then
| I0 = | 1 | Σ(Iab) = | Σ(Iab) | Imax |
| 2R | 2RImax |
where Imax stands for the maximum candle-power of the arc. If, then, we set off at b a line bH perpendicular to DE and in length proportional to the candle-power of the arc in the direction AP, and carry out the same construction for a number of different observed candle-power readings at known angles above and below the horizon, the summits of all ordinates such as bH will define a curve DHE. The mean spherical candle-power of the arc is equal to the product of the maximum candle-power (Imax), and a fraction equal to the ratio of the area included by the curve DHE to its circumscribing rectangle DFGE. The area of the curve DHE multiplied by 2π/R gives us the total flux of light from the arc.
Owing to the inequality in the distribution of light from an electric arc, it is impossible to define the illuminating power by a single number in any other way than by stating the mean spherical candle-power. All such commonly used expressions as “an arc lamp of 2000 candle-power” are, therefore, perfectly meaningless.
The photometry of arc lamps presents particular difficulties, owing to the great difference in quality between the light radiated by the arc and that given by any of the ordinarily used light standards. (For standards of light and Photometry of arc. photometers, see Photometer.) All photometry depends on the principle that if we illuminate two white surfaces respectively and exclusively by two separate sources of light, we can by moving the lights bring the two surfaces into such a condition that their illumination or brightness is the same without regard to any small colour difference. The quantitative measurement depends on the fact that the illumination produced upon a surface by a source of light is inversely as the square of the distance of the source. The trained eye is capable of making a comparison between two surfaces illuminated by different sources of light, and pronouncing upon their equality or otherwise in respect of brightness, apart from a certain colour difference; but for this to be done with accuracy the two illuminated surfaces, the brightness of which is to be compared, must be absolutely contiguous and not separated by any harsh line. The process of comparing the light from the arc directly with that of a candle or other similar flame standard is exceedingly difficult, owing to the much greater proportion and intensity of the violet rays in the arc. The most convenient practical working standard is an incandescent lamp run at a high temperature, that is, at an efficiency of about 2½ watts per candle. If it has a sufficiently large bulb, and has been aged by being worked for some time previously, it will at a constant voltage preserve a constancy in illuminating power sufficiently long to make the necessary photometric comparisons, and it can itself be compared at intervals with another standard incandescent lamp, or with a flame standard such as a Harcourt pentane lamp.
 |
| Fig. 10. |
In measuring the candle-power of arc lamps it is necessary to have some arrangement by which the brightness of the rays proceeding from the arc in different directions can be measured. For this purpose the lamp may be suspended from a support, and a radial arm arranged to carry three mirrors, so that in whatever position the arm may be placed, it gathers light proceeding at one particular angle above or below the horizon from the arc, and this light is reflected out finally in a constant horizontal direction. An easily-arranged experiment enables us to determine the constant loss of light by reflection at all the mirrors, since that reflection always takes place at 45°. The ray thrown out horizontally can then be compared with that from any standard source of light by means of a fixed photometer, and by sweeping round the radial arm the photometric or illuminating curve of the arc lamp can be obtained. From this we can at once determine the nature of the illumination which would be produced on a horizontal surface if the arc lamp were suspended at a given distance above it. Let A (fig. 10) be an arc lamp placed at a height h( = AB) above a horizontal plane. Let ACD be the illuminating power curve of the arc, and hence AC the candle-power in a direction AP. The illumination (I) or brightness on the horizontal plane at P is equal to
AC cos APM/(AP)2 = FC/(h2 + x2), where x = BP.
 |
| Fig. 11. |
Hence if the candle-power curve of the arc and its height above the surface are known, we can describe a curve BMN, whose ordinate PM will denote the brightness on the horizontal surface at any point P. It is easily seen that this ordinate must have a maximum value at some point. This brightness is best expressed in candle-feet, taking the unit of illumination to be that given by a standard candle on a white surface at a distance of 1 ft. If any number of arc lamps are placed above a horizontal plane, the brightness at any point can be calculated by adding together the illuminations due to each respectively.
The process of delineating the photometric or polar curve of intensity for an arc lamp is somewhat tedious, but the curve has the advantage of showing exactly the distribution of light in different directions. When only the mean spherical or mean hemispherical candle-power is required the process can be shortened by employing an integrating photometer such as that of C. P. Matthews (Trans. Amer. Inst. Elec. Eng., 1903, 19, p. 1465), or the lumen-meter of A. E. Blondel which enables us to determine at one observation the total flux of light from the arc and therefore the mean spherical candle-power per watt.
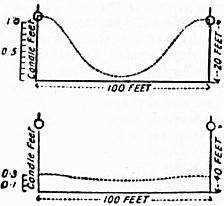 |
| Fig. 12. |
In the use of arc lamps for street and public lighting, the question of the distribution of light on the horizontal surface is all-important. In order that street surfaces may be well lighted, the minimum illumination should Street arc lighting. not fall below 0.1 candle-foot, and in general, in well-lighted streets, the maximum illumination will be 1 candle-foot and upwards. By means of an illumination photometer, such as that of W. H. Preece and A. P. Trotter, it is easy to measure the illumination in candle-feet at any point in a street surface, and to plot out a number of contour lines of equal illumination. Experience has shown that to obtain satisfactory results the lamps must be placed on a high mast 20 or 25 ft. above the roadway surface. These posts are now generally made of cast iron in various ornamental forms (fig. 11), the necessary conductors for conveying the current up to the lamp being taken inside the iron mast. (The pair of incandescent lamps halfway down the standard are for use in the middle of the night, when the arc lamp would give more light than is required; they are lighted by an automatic switch whenever the arc is extinguished.) The lamp itself is generally enclosed in an opalescent spherical globe, which is woven over with wire-netting so that in case of fracture the pieces may not cause damage. The necessary trimming, that is, the replacement of carbons, is effected either by lowering the lamp or, preferably, by carrying round a portable ladder enabling the trimmer to reach it. For the purpose of public illumination it is very usual to employ a lamp taking 10 amperes, and therefore absorbing about 500 watts. Such a lamp is called a 500-watt arc lamp, and it is found that a satisfactory illumination is given for most street purposes by placing 500-watt arc lamps at distances varying from 40 to 100 yds., and at a height of 20 to 25 ft. above the roadway. The maximum candle-power of a 500-watt arc enclosed in a roughened or ground-glass globe will not exceed 1500 candles, and that of a 6.8-ampere arc (continuous) about 900 candles. If, however, the arc is an enclosed arc with double globes, the absorption of light would reduce the effective maximum to about 200 c.p. and 120 c.p. respectively. When arc lamps are placed in public thoroughfares not less than 40 yds. apart, the illumination anywhere on the street surface is practically determined by the two nearest ones. Hence the total illumination at any point may be obtained by adding together the illuminations due to each arc separately. Given the photometric polar curves or illuminating-power curves of each arc taken outside the shade or globe, we can therefore draw a curve representing the resultant illumination on the horizontal surface. It is obvious that the higher the lamps are placed, the more uniform is the street surface illumination, but the less its average value; thus two 10-ampere arcs placed on masts 20 ft. above the road surface and 100 ft. apart will give a maximum illumination of about 1.1 and a minimum of about 0.15 candle-feet in the interspace (fig 12). If the lamps are raised on 40-ft. posts the maximum illumination will fall to 0.3, and the minimum will rise to 0.2. For this reason masts have been employed as high as 90 ft. In docks and railway yards high masts (50 ft.) are an advantage, because the strong contrasts due to shadows of trucks, carts, &c., then become less marked, but for street illumination they should not exceed 30 to 35 ft. in height. Taking the case of 10-ampere and 6.8-ampere arc lamps in ordinary opal shades, the following figures have been given by Trotter as indicating the nature of the resultant horizontal illumination:—
| Arc Current in Amperes. |
Height above Road in Feet. |
Distance apart in Feet. |
Horizontal Illumination in Candle-Feet. |
|
| Maximum. | Minimum. | |||
| 10 | 20 | 120 | 1.85 | 0.12 |
| 10 | 25 | 120 | 1.17 | 0.15 |
| 10 | 40 | 120 | 0.5 | 0.28 |
| 6.8 | 20 | 90 | 1.1 | 0.21 |
| 6.8 | 40 | 120 | 0.3 | 0.17 |
As regards distance apart, a very usual practice is to place the lamps at spaces equal to six to ten times their height above the road surface. Blondel (Electrician, 35, p. 846) gives the following rule for the height (h) of the arc to afford the maximum illumination at a distance (d) from the foot of the lamp-post, the continuous current arc being employed:—
| For naked arc | h = 0.95 d. |
| ” arc in rough glass globe | h = 0.85 d. |
| ” ” opaline glob | h = ” |
| ” ” opal globe | h = 0.5 d. |
| ” ” holophane globe | h = 0.5 d. |
These figures show that the distribution of light on the horizontal surface is greatly affected by the nature of the enclosing globe. For street illumination naked arcs, although sometimes employed in works and factory yards, are entirely unsuitable, since the result produced on the eye by the bright point of light is to paralyse a part of the retina and contract the pupil, hence rendering the eye less sensitive when directed on feebly illuminated surfaces. Accordingly, diffusing globes have to be employed. It is usual to place the arc in the interior of a globe of from 12 to 18 in. in diameter. This may be made of ground glass, opal glass, or be a dioptric globe such as the holophane. The former two are strongly absorptive, as may be seen from the results of experiments by Guthrie and Redhead. The following table shows the astonishing loss of light due to the use of opal globes:—
| Naked Arc. |
Arc in Clear Globe. |
Arc in Rough Glass Globe. |
Arc in Opal Globe. |
|
| Mean spherical c.p. | 319 | 235 | 160 | 144 |
| Mean hemispherical c.p. | 450 | 326 | 215 | 138 |
| Percentage value of transmitted light | 100 | 53 | 23 | 19 |
| Percentage absorption | 0 | 47 | 77 | 81 |
By using Trotter’s, Fredureau’s or the holophane globe, the light may be so diffused that the whole globe appears uniformly luminous, and yet not more than 20% of the light is absorbed. Taking the absorption of an ordinary opal globe into account, a 500-watt arc does not usually give more than 500 c.p. as a maximum candle-power. Even with a naked 500-watt arc the mean spherical candle-power is not generally more than 500 c.p., or at the rate of 1 c.p. per watt. The maximum candle-power for a given electrical power is, however, greatly dependent on the current density in the carbon, and to obtain the highest current density the carbons must be as thin as possible. (See T. Hesketh, “Notes on the Electric Arc,” Electrician, 39, p. 707.)
For the efficiency of arcs of various kinds, expressed by the mean hemispherical candle power per ampere and per watt expended in the arc, the following figures were given by L. Andrews (“Long-flame Arc Lamps,” Journal Inst. Elec. Eng., 1906, 37, p. 4).
| Candle-power per ampere. |
Candle-power per watt. |
|
| Ordinary open carbon arc | 82 | 1.54 |
| Enclosed carbon arc | 55 | 0.77 |
| Chemical carbon or flame arc | 259 | 5.80 |
| High voltage inclined carbon arc | 200 | 2.24 |
It will be seen that the flame arc lamp has an enormous advantage over other types in the light yielded for a given electric power consumption.
The practical employment of the electric arc as a means of illumination is dependent upon mechanism for automatically keeping two suitable carbon rods in the proper position, and moving them so as to enable a steady arc to be Arc lamp mechanism. maintained. Means must be provided for holding the carbons in line, and when the lamp is not in operation they must fall together, or come together when the current is switched on, so as to start the arc. As soon as the current passes, they must be moved slightly apart, and gripped in position immediately the current reaches its right value, being moved farther apart if the current increases in strength, and brought together if it decreases. Moreover, it must be possible for a considerable length of carbon to be fed through the lamp as required.
 |
| Fig. 13 |
 |
| Fig. 14 |
One early devised form of arc-lamp mechanism was a system of clockwork driven by a spring or weight, which was started and stopped by the action of an electromagnet; in modern lighthouse lamps a similar mechanism is still employed. W. E. Staite (1847), J. B. L. Foucault (1849), V. L. M. Serrin (1857), J. Duboscq (1858), and a host of later inventors, devised numerous forms of mechanical and clockwork lamps. The modern self-regulating type may be said to have been initiated in 1878 by the differential lamp of F. von Hefner-Alteneck, and the clutch lamp of C. F. Brush. The general principle of the former may be explained as follows: There are two solenoids, placed one above the other. The lower one, of thick wire, is in series with the two carbon rods forming the arc, and is hence called the series coil. Above this there is placed another solenoid of fine wire, which is called the shunt coil. Suppose an iron rod to be placed so as to be partly in one coil and partly in another; then when the coils are traversed by currents, the iron core will be acted upon by forces tending to pull it into these solenoids. If the iron core be attached to one end of a lever, the other end of which carries the upper carbon, it will be seen that if the carbons are in contact and the current is switched on, the series coil alone will be traversed by the current, and its magnetic action will draw down the iron core, and therefore pull the carbons apart and strike the arc. The moment the carbons separate, there will be a difference of potential between them, and the shunt coil will then come into action, and will act on the core so as to draw the carbons together. Hence the two solenoids act in opposition to each other, one increasing and the other diminishing the length of the arc, and maintaining the carbons in the proper position. In the lamp of this type the upper carbon is in reality attached to a rod having a side-rack gearing, with a train of wheels governed by a pendulum. The action of the series coil on the mechanism is to first lock or stop the train, and then lift it as a whole slightly. This strikes the arc. When the arc is too long, the series coil lowers the gear and finally releases the upper carbon, so that it can run down by its own weight. The principle of a shunt and series coil operating on an iron core in opposition is the basis of the mechanism of a number of arc lamps. Thus the lamp invented by F. Krizik and L. Piette, called from its place of origin the Pilsen lamp, comprises an iron core made in the shape of a double cone or spindle (fig. 13), which is so arranged in a brass tube that it can move into or out of a shunt and series coil, wound the one with fine and the other with thick insulated wire, and hence regulate the position of the carbon attached to it. The movement of this core is made to feed the carbons directly without the intervention of any clockwork, as in the case of the Hefner-Alteneck lamp. In the clutch-lamp mechanism the lower carbon is fixed, and the upper carbon rests upon it by its own weight and that of its holder. The latter consists of a long rod passing through guides, and is embraced somewhere by a ring capable of being tilted or lifted by a finger attached to the armature of an electromagnet the coils of which are in series with the arc. When the current passes through the magnet it attracts the armature, and by tilting the ring lifts the upper carbon-holder and hence strikes the arc. If the current diminishes in value, the upper carbon drops a little by its own weight, and the feed of the lamp is thus effected by a series of small lifts and drops of the upper carbon (fig. 14). Another element sometimes employed in arc-lamp mechanism is the brake-wheel regulator. This is a feature of one form of the Brockie and of the Crompton-Pochin lamps. In these the movement of the carbons is effected by a cord or chain which passes over a wheel, or by a rack geared with the brake wheel. When no current is passing through the lamp, the wheel is free to move, and the carbons fall together; but when the current is switched on, the chain or cord passing over the brake wheel, or the brake wheel itself is gripped in some way, and at the same time the brake wheel is lifted so that the arc is struck.
Although countless forms of self-regulating device have been invented for arc lamps, nothing has survived the test of time so well as the typical mechanisms which work with carbon rods in one line, one or both rods being moved by a controlling apparatus as required. The early forms of semi-incandescent arc lamp, such as those of R. Werdermann and others, have dropped out of existence. These were not really true arc lamps, the light being produced by the incandescence of the extremity of a thin carbon rod pressed against a larger rod or block. The once famous Jablochkoff candle, invented in 1876, consisted of two carbon rods about 4 mm. in diameter, placed parallel to each other and separated by a partition of kaolin, steatite or other refractory non-conductor. Alternating currents were employed, and the candle was set in operation by a match or starter of high-resistance carbon paste which connected the tips of the rods. When this burned off, a true arc was formed between the parallel carbons, the separator volatilizing as the carbons burned away. Although much ingenuity was expended on this system of lighting between 1877 and 1881, it no longer exists. One cause of its disappearance was its relative inefficiency in light-giving power compared with other forms of carbon arc taking the same amount of power, and a second equally important reason was the waste in carbons. If the arc of the electric candle was accidentally blown out, no means of relighting existed; hence the great waste in half-burnt candles. H. Wilde, J. C. Jamin, J. Rapieff and others endeavoured to provide a remedy, but without success.
It is impossible to give here detailed descriptions of a fraction of the arc-lamp mechanisms devised, and it must suffice to indicate the broad distinctions between various types. (1) Arc lamps may be either continuous-current or alternating-current lamps. For outdoor public illumination the former are greatly preferable, as owing to the form of the illuminating power-curve they send the light down on the road surface, provided the upper carbon is the positive one. For indoor, public room or factory lighting, inverted arc lamps are sometimes employed. In this case the positive carbon is the lower one, and the lamp is carried in an inverted metallic reflector shield, so that the light is chiefly thrown up on the ceiling, whence it is diffused all round. The alternating-current arc is not only less efficient in mean spherical candle-power per watt of electric power absorbed, but its distribution of light is disadvantageous for street purposes. Hence when arc lamps have to be worked off an alternating-current circuit for public lighting it is now usual to make use of a rectifier, which rectifies the alternating current into an unidirectional though pulsating current. (2.) Arc lamps may be also classified, as above described, into open or enclosed arcs. The enclosed arc can be made to burn for 200 hours with one pair of carbons, whereas open-arc lamps are usually only able to work, 8, 16 or 32 hours without recarboning, even when fitted with double carbons. (3) Arc lamps are further divided into focussing and non-focussing lamps. In the former the lower carbon is made to move up as the upper carbon moves down, and the arc is therefore maintained at the same level. This is advisable for arcs included in a globe, and absolutely necessary in the case of lighthouse lamps and lamps for optical purposes. (4) Another subdivision is into hand-regulated and self-regulating lamps. In the hand-regulated arcs the carbons are moved by a screw attachment as required, as in some forms of search-light lamp and lamps for optical lanterns. The carbons in large search-light lamps are usually placed horizontally. The self-regulating lamps may be classified into groups depending upon the nature of the regulating appliances. In some cases the regulation is controlled only by a series coil, and in others only by a shunt coil. Examples of the former are the original Gülcher and Brush clutch lamp, and some modern enclosed arc lamps; and of the latter, the Siemens “band” lamp, and the Jackson-Mensing lamp. In series coil lamps the variation of the current in the coil throws into or out of action the carbon-moving mechanism; in shunt coil lamps the variation in voltage between the carbons is caused to effect the same changes. Other types of lamp involve the use both of shunt and series coils acting against each other. A further classification of the self-regulating lamps may be found in the nature of the carbon-moving mechanism. This may be some modification of the Brush ring clutch, hence called clutch lamps; or some variety of brake wheel, as employed in Brockie and Crompton lamps; or else some form of electric motor is thrown into or out of action and effects the necessary changes. In many cases the arc-lamp mechanism is provided with a dash-pot, or contrivance in which a piston moving nearly air-tight in a cylinder prevents sudden jerks in the motion of the mechanism, and thus does away with the “hunting” or rapid up-and-down movements to which some varieties of clutch mechanism are liable. One very efficient form is illustrated in the Thomson lamp and Brush-Vienna lamp. In this mechanism a shunt and series coil are placed side by side, and have iron cores suspended to the ends of a rocking arm held partly within them. Hence, according as the magnetic action of the shunt or series coil prevails, the rocking arm is tilted backwards or forwards. When the series coil is not in action the motion is free, and the upper carbon-holder slides down, or the lower one slides up, and starts the arc. The series coil comes into action to withdraw the carbons, and at the same time locks the mechanism. The shunt coil then operates against the series coil, and between them the carbon is fed forwards as required. The control to be obtained is such that the arc shall never become so long as to flicker and become extinguished, when the carbons would come together again with a rush, but the feed should be smooth and steady, the position of the carbons responding quickly to each change in the current.
The introduction of enclosed arc lamps was a great improvement, in consequence of the economy effected in the consumption of carbon and in the cost of labour for trimming. A well-known and widely used form of enclosed arc lamp is the Jandus lamp, which in large current form can be made to burn for two hundred hours without recarboning, and in small or midget form to burn for forty hours, taking a current of two amperes at 100 volts. Such lamps in many cases conveniently replace large sizes of incandescent lamps, especially for shop lighting, as they give a whiter light. Great improvements have also been made in inclined carbon arc lamps. One reason for the relatively low efficiency of the usual vertical rod arrangement is that the crater can only radiate laterally, since owing to the position of the negative carbon no crater light is thrown directly downwards. If, however, the carbons are placed in a downwards slanting position at a small angle like the letter V and the arc formed at the bottom tips, then the crater can emit downwards all the light it produces. It is found, however, that the arc is unsteady unless a suitable magnetic field is employed to keep the arc in position at the carbon tips. This method has been adopted in the Carbone arc, which, by the employment of inclined carbons, and a suitable electromagnet to keep the true arc steady at the ends of the carbons, has achieved considerable success. One feature of the Carbone arc is the use of a relatively high voltage between the carbons, their potential difference being as much as 85 volts.
Arc lamps may be arranged either (i.) in series, (ii.) in parallel or (iii.) in series parallel. In the first case a number, say 20, may be traversed by the same current, in that case supplied at a pressure of 1000 volts. Each must have Arrangement. a magnetic cut-out, so that if the carbons stick together or remain apart the current to the other lamps is not interrupted, the function of such a cut-out being to close the main circuit immediately any one lamp ceases to pass current. Arc lamps worked in series are generally supplied with a current from a constant current dynamo, which maintains an invariable current of, say 10 amperes, independently of the number of lamps on the external circuit. If the lamps, however, are worked in series off a constant potential circuit, such as one supplying at the same time incandescent lamps, provision must be made by which a resistance coil can be substituted for any one lamp removed or short-circuited. When lamps are worked in parallel, each lamp is independent, but it is then necessary to add a resistance in series with the lamp. By special devices three lamps can be worked in series of 100 volt circuits. Alternating-current arc lamps can be worked off a high-tension circuit in parallel by providing each lamp with a small transformer. In some cases the alternating high-tension current is rectified and supplied as a unidirectional current to lamps in series. If single alternating-current lamps have to be worked off a 100 volt alternating-circuit, each lamp must have in series with it a choking coil or economy coil, to reduce the circuit pressure to that required for one lamp. Alternating-current lamps take a larger effective current, and work with a less effective or virtual carbon P.D., than continuous current arcs of the same wattage.
The cost of working public arc lamps is made up of several items. There is first the cost of supplying the necessary electric energy, then the cost of carbons and the labour of recarboning, and, lastly, an item due to depreciation Cost. and repairs of the lamps. An ordinary type of open 10 ampere arc lamp, burning carbons 15 and 9 mm. in diameter for the positive and negative, and working every night of the year from dusk to dawn, uses about 600 ft. of carbons per annum. If the positive carbon is 18 mm. and the negative 12 mm., the consumption of each size of carbon is about 70 ft. per 1000 hours of burning. It may be roughly stated that at the present prices of plain open arc-lamp carbons the cost is about 15s. per 1000 hours of burning; hence if such a lamp is burnt every night from dusk to midnight the annual cost in that respect is about £1, 10s. The annual cost of labour per lamp for trimming is in Great Britain from £2 to £3; hence, approximately speaking, the cost per annum of maintenance of a public arc lamp burning every night from dusk to midnight is about £4 to £5, or perhaps £6, per annum, depreciation and repairs included. Since such a 10 ampere lamp uses half a Board of Trade unit of electric energy every hour, it will take 1000 Board of Trade units per annum, burning every night from dusk to midnight; and if this energy is supplied, say at 1½d. per unit, the annual cost of energy will be about £6, and the upkeep of the lamp, including carbons, labour for trimming and repairs, will be about £10 to £11 per annum. The cost for labour and carbons is considerably reduced by the employment of the enclosed arc lamp, but owing to the absorption of light produced by the inner enclosing globe, and the necessity for generally employing a second outer globe, there is a lower resultant candle-power per watt expended in the arc. Enclosed arc lamps are made to burn without attention for 200 hours, singly on 100 volt circuits, or two in series on 200 volt circuits, and in addition to the cost of carbons per hour being only about one-twentieth of that of the open arc, they have another advantage in the fact that there is a more uniform distribution of light on the road surface, because a greater proportion of light is thrown out horizontally.
It has been found by experience that the ordinary type of open arc lamp with vertical carbons included in an opalescent globe cannot compete in point of cost with modern improvements in gas lighting as a means of street illumination. The violet colour of the light and the sharp shadows, and particularly the non-illuminated area just beneath the lamp, are grave disadvantages. The high-pressure flame arc lamp with inclined chemically treated carbons has, however, put a different complexion on matters. Although the treated carbons cost more than the plain carbons, yet there is a great increase of emitted light, and a 9-ampere flame arc lamp supplied with electric energy at 1½d. per unit can be used for 1000 hours at an inclusive cost of about £s to £6, the mean emitted illumination being at the rate of 4 c.p. per watt absorbed. In the Carbone arc lamp, the carbons are worked at an angle of 15° or 20° to each other and the arc is formed at the lower ends. If the potential difference of the carbons is low, say only 50-60 volts, the crater forms between the tips of the carbons and is therefore more or less hidden. If, however, the voltage is increased to 90-100 then the true flame of the arc is longer and is curved, and the crater forms at the exteme tip of the carbons and throws all its light downwards. Hence results a far greater mean hemispherical candle power (M.H.S.C.P.), so that whereas a 10-ampere 60 volt open arc gives at most 1200 M.H.S.C.P., a Carbone 10-ampere 85 volt arc will give 2700 M.H.S.C.P. Better results still can be obtained with impregnated carbons. But the flame arcs with impregnated carbons cannot be enclosed, so the consumption of carbon is greater, and the carbons themselves are more costly, and leave a greater ash on burning; hence more trimming is required. They give a more pleasing effect for street lighting, and their golden yellow globe of light is more useful than an equally costly plain arc of the open type. This improvement in efficiency is, however, accompanied by some disadvantages. The flame arc is very sensitive to currents of air and therefore has to be shielded from draughts by putting it under an “economizer” or chamber of highly refractory material which surrounds the upper carbon, or both carbon tips, if the arc is formed with inclined carbons. (For additional information on flame arc lamps see a paper by L. B. Marks and H. E. Clifford, Electrician, 1906, 57, p. 975.)
2. Incandescent Lamps.—Incandescent electric lighting, although not the first, is yet in one sense the most obvious method of utilizing electric energy for illumination. It was evolved from the early observed fact that a conductor is heated when traversed by an electric current, and that if it has a high resistance and a high melting-point it may be rendered incandescent, and therefore become a source of light. Naturally every inventor turned his attention to the employment of wires of refractory metals, such as platinum or alloys of platinum-iridium, &c., for the purpose of making an incandescent lamp. F. de Moleyns experimented in 1841, E. A. King and J. W. Starr in 1845, J. J. W. Watson in 1853, and W. E. Staite in 1848, but these inventors achieved no satisfactory result. Part of their want of success is attributable to the fact that the problem of the economical production of electric current by the dynamo machine had not then been solved. In 1878 T. A. Edison devised lamps in which a platinum wire was employed as the light-giving agent, carbon being made to adhere round it by pressure. Abandoning this, he next directed his attention to the construction of an “electric candle,” consisting of a thin cylinder or rod formed of finely-divided metals, platinum, iridium, &c., mixed with refractory oxides, such as magnesia, or zirconia, lime, &c. This refractory body was placed in a closed vessel and heated by being traversed by an electric current. In a further improvement he proposed to use a block of refractory oxide, round which a bobbin of fine platinum or platinum-iridium wire was coiled. Every other inventor who worked at the problem of incandescent lighting seems to have followed nearly the same path of invention. Long before this date, however, the notion of employing carbon as a substance to be heated by the current had entered the minds of inventors; even in 1845 King had employed a small rod of plumbago as the substance to be heated. It was obvious, however, that carbon could only be so heated when in a space destitute of oxygen, and accordingly King placed his plumbago rod in a barometric vacuum. S. W. Konn in 1872, and S. A. Kosloff in 1875, followed in the same direction.
No real success attended the efforts of inventors until it was finally recognized, as the outcome of the work by J. W. Swan, T. A. Edison, and, in a lesser degree, St. G. Lane Fox and W. E. Sawyer and A. Man, that the conditions Carbon filament lamp. of success were as follow: First, the substance to be heated must be carbon in the form of a thin wire rod or thread, technically termed a filament; second, this must be supported and enclosed in a vessel formed entirely of glass; third, the vessel must be exhausted as perfectly as possible; and fourth, the current must be conveyed into and out of the carbon filament by means of platinum wires hermetically sealed through the glass.
One great difficulty was the production of the carbon filament. King, Sawyer, Man and others had attempted to cut out a suitably shaped piece of carbon from a solid block; but Edison and Swan were the first to show that the proper solution of the difficulty was to carbonize an organic substance to which the necessary form had been previously given. For this purpose cardboard, paper and ordinary thread were originally employed, and even, according to Edison, a mixture of lampblack and tar rolled out into a fine wire and bent into a spiral. At one time Edison employed a filament of bamboo, carbonized after being bent into a horse-shoe shape. Swan used a material formed by treating ordinary crochet cotton-thread with dilute sulphuric acid, the “parchmentized thread” thus produced being afterwards carbonized. In the modern incandescent lamp the filament is generally constructed by preparing first of all a form of soluble cellulose. Carefully purified cotton-wool is dissolved in some solvent, such as a solution of zinc chloride, and the viscous material so formed is forced by hydraulic pressure through a die. The long thread thus obtained, when hardened, is a semi-transparent substance resembling cat-gut, and when carefully carbonized at a high temperature gives a very dense and elastic form of carbon filament. It is cut into appropriate lengths, which after being bent into horse-shoes, double-loops, or any other shape desired, are tied or folded round carbon formers and immersed in plumbago crucibles, packed in with finely divided plumbago. The crucibles are then heated to a high temperature in an ordinary combustion or electric furnace, whereby the organic matter is destroyed, and a skeleton of carbon remains. The higher the temperature at which this carbonization is conducted, the denser is the resulting product. The filaments so prepared are sorted and measured, and short leading-in wires of platinum are attached to their ends by a carbon cement or by a carbon depositing process, carried out by heating electrically the junction of the carbon and platinum under the surface of a hydrocarbon liquid. They are then mounted in bulbs of lead glass having the same coefficient of expansion as platinum, through the walls of which, therefore, the platinum wires can be hermetically sealed. The bulbs pass into the exhausting-room, where they are exhausted by some form of mechanical or mercury pump. During this process an electric current is sent through the filament to heat it, in order to disengage the gases occluded in the carbon, and exhaustion must be so perfect that no luminous glow appears within the bulb when held in the hand and touched against one terminal of an induction coil in operation.
In the course of manufacture a process is generally applied to the carbon which is technically termed “treating.” The carbon filament is placed in a vessel surrounded by an atmosphere of hydrocarbon, such as coal gas or vapour of benzol. If current is then passed through the filament the hydrocarbon vapour is decomposed, and carbon is thrown down upon the filament in the form of a lustrous and dense deposit having an appearance like steel when seen under the microscope. This deposited carbon is not only much more dense than ordinary carbonized organic material, but it has a much lower specific electric resistance. An untreated carbon filament is generally termed the primary carbon, and a deposited carbon the secondary carbon. In the process of treating, the greatest amount of deposit is at any places of high resistance in the primary carbon, and hence it tends to cover up or remedy the defects which may exist. The bright steely surface of a well-treated filament is a worse radiator than the rougher black surface of an untreated one; hence it does not require the expenditure of so much electric power to bring it to the same temperature, and probably on account of its greater density it ages much less rapidly.
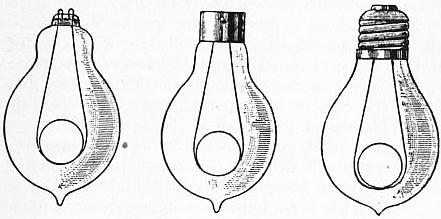 |
| Fig. 15. |
 |
| Fig. 16.—Incandescent Lamp Sockets. |
Finally, the lamp is provided with a collar having two sole plates on it, to which the terminal wires are attached, or else the terminal wires are simply bent into two loops; in a third form, the Edison screw terminal, it is provided with a central metal plate, to which one end of the filament is connected, the other end being joined to a screw collar. The collars and screws are formed of thin brass embedded in plaster of Paris, or in some material like vitrite or black glass (fig. 15). To put the lamp into connexion with the circuit supplying the current, it has to be fitted into a socket or holder. Three of the principal types of holder in use are the bottom contact (B.C.) or Dornfeld socket, the Edison screw-collar socket and the Swan or loop socket. In the socket of C. Dornfeld (fig. 16, a and a′) two spring pistons, in contact with the two sides of the circuit, are fitted into the bottom of a short metallic tube having bayonet joint slots cut in the top. The brass collar on the lamp has two pins, by means of which a bayonet connexion is made between it and the socket; and when this is done, the spring pins are pressed against the sole plates on the lamp. In the Edison socket (fig. 16, b) a short metal tube with an insulating lining has on its interior a screw sleeve, which is in connexion with one wire of the circuit; at the bottom of the tube, and insulated from the screw sleeve, is a central metal button, which is in connexion with the other side of the circuit. On screwing the lamp into the socket, the screw collar of the lamp and the boss or plate at the base of the lamp make contact with the corresponding parts of the socket, and complete the connexion. In some cases a form of switch is included in the socket, which is then termed the key-holder. For loop lamps the socket consists of an insulated block, having on it two little hooks, which engage with the eyes of the lamp. This insulating block also carries some form of spiral spring or pair of spring loops, by means of which the lamp is pressed away from the socket, and the eyes kept tight by the hooks. This spring or Swan socket (fig. 16, c) is found useful in places where the lamps are subject to vibration, for in such cases the Edison screw collar cannot well be used, because the vibration loosens the contact of the lamp in the socket. The sockets may be fitted with appliances for holding ornamental shades or conical reflectors.
The incandescent filament being a very brilliant line of light, various devices are adopted for moderating its brilliancy and distributing the light. A simple method is to sand-blast the exterior of the bulb, whereby it acquires an appearance similar to that of ground glass, or the bare lamp may be enclosed in a suitable glass shade. Such shades, however, if made of opalescent or semi-opaque glass, absorb 40 to 60% of the light; hence various forms of dioptric shade have been invented, consisting of clear glass ruled with prismatic grooves in such a manner as to diffuse the light without any very great absorption. Invention has been fertile in devising etched, coloured, opalescent, frosted and ornamental shades for decorative purposes, and in constructing special forms for use in situations, such as mines and factories for explosives, where the globe containing the lamp must be air-tight. High candle-power lamps, 500, 1000 and upwards, are made by placing in one large glass bulb a number of carbon filaments arranged in parallel between two rings, which are connected with the main leading-in wires. When incandescent lamps are used for optical purposes it is necessary to compress the filament into a small space, so as to bring it into the focus of a lens or mirror. The filament is then coiled or crumpled up into a spiral or zigzag form. Such lamps are called focus lamps.
Incandescent lamps are technically divided into high and low voltage lamps, high and low efficiency lamps, standard and fancy lamps. The difference between high and low efficiency lamps is based upon the relation of the Classification of lamps. power absorbed by the lamp to the candle-power emitted. Every lamp when manufactured is marked with a certain figure, called the marked volts. This is understood to be the electromotive force in volts which must be applied to the lamp terminals to produce through the filament a current of such magnitude that the lamp will have a practically satisfactory life, and give in a horizontal direction a certain candle-power, which is also marked upon the glass. The numerical product of the current in amperes passing through the lamp, and the difference in potential of the terminals measured in volts, gives the total power taken up by the lamp in watts; and this number divided by the candle-power of the lamp (taking generally a horizontal direction) gives the watts per candle-power. This is an important figure, because it is determined by the temperature; it therefore determines the quality of the light emitted by the lamp, and also fixes the average duration of the filament when rendered incandescent by a current. Even in a good vacuum the filament is not permanent. Apart altogether from accidental defects, the carbon is slowly volatilized, and carbon molecules are also projected in straight lines from different portions of the filament. This process not only causes a change in the nature of the surface of the filament, but also a deposit of carbon on the interior of the bulb, whereby the glass is blackened and the candle-power of the lamp reduced. The volatilization increases very rapidly as the temperature rises. Hence at points of high resistance in the filament, more heat being generated, a higher temperature is attained, and the scattering of the carbon becomes very rapid; in such cases the filament is sooner or later cut through at the point of high resistance. In order that incandescent lighting may be practically possible, it is essential that the lamps shall have a certain average life, that is, duration; and this useful duration is fixed not merely by the possibility of passing a current through the lamp at all, but by the rate at which the candle-power diminishes. The decay of candle-power is called the ageing of the lamp, and the useful life of the lamp may be said to be that period of its existence before it has deteriorated to a point when it gives only 75% of its original candle-power. It is found that in practice carbon filament lamps, as at present made, if worked at a higher efficiency than 2½ watts per candle-power, exhibit a rapid deterioration in candle-power and an abbreviated life. Hence lamp manufacturers classify lamps into various classes, marked for use say at 2½, 3, 3½ and 4 watts per candle. A 2½ watt per candle lamp would be called a high-efficiency lamp, and a 4 watt per candle lamp would be called a low-efficiency lamp. In ordinary circumstances the low-efficiency lamp would probably have a longer life, but its light would be less suitable for many purposes of illumination in which colour discrimination is required.
The possibility of employing high-efficiency lamps depends greatly on the uniformity of the electric pressure of the supply. If the voltage is exceedingly uniform, then high-efficiency lamps can be satisfactorily employed; but they are not adapted for standing the variations in pressure which are liable to occur with public supply-stations, since, other things being equal, their filaments are less substantial. The classification into high and low voltage lamps is based upon the watts per candle-power corresponding to the marked volts. When incandescent lamps were first introduced, the ordinary working voltage was 50 or 100, but now a large number of public supply-stations furnish current to consumers at a pressure of 200 or 250 volts. This increase was necessitated by the enlarging area of supply in towns, and therefore the necessity for conveying through the same subterranean copper cables a large supply of electric energy without increasing the maximum current value and the size of the cables. This can only be done by employing a higher working electromotive force; hence arose a demand for incandescent lamps having marked volts of 200 and upwards, technically termed high-voltage lamps. The employment of higher pressures in public supply-stations has necessitated greater care in the selection of the lamp fittings, and in the manner of carrying out the wiring work. The advantages, however, of higher supply pressures, from the point of view of supply-stations, are undoubted. At the same time the consumer desired a lamp of a higher efficiency than the ordinary carbon filament lamp. The demand for this stimulated efforts to produce improved carbon lamps, and it was found that if the filament were exposed to a very high temperature, 3000° C. in an electric furnace, it became more refractory and was capable of burning in a lamp at an efficiency of 2½ watts per c.p. Inventors also turned their attention to substances other than carbon which can be rendered incandescent by the electric current.
The luminous efficiency of any source of light, that is to say, the percentage of rays emitted which affect the eye as light compared with the total radiation, is dependent upon its temperature. In an ordinary oil lamp the luminous Oxide filaments. rays do not form much more than 3% of the total radiation. In the carbon-filament incandescent lamp, when worked at about 3 watts per candle, the luminous efficiency is about 5%; and in the arc lamp the radiation from the crater contains about 10 to 15% of eye-affecting radiation. The temperature of a carbon filament working at about 3 watts per candle is not far from the melting-point of platinum, that is to say, is nearly 1775° C. If it is worked at a higher efficiency, say 2.5 watts per candle-power, the temperature rises rapidly, and at the same time the volatilization and molecular scattering of the carbon is rapidly increased, so that the average duration of the lamp is very much shortened. An improvement, therefore, in the efficiency of the incandescent lamp can only be obtained by finding some substance which will endure heating to a higher temperature than the carbon filament. Inventors turned their attention many years ago, with this aim, to the refractory oxides and similar substances. Paul Jablochkoff in 1877 described and made a lamp consisting of a piece of kaolin, which was brought to a state of incandescence first by passing over it an electric spark, and afterwards maintained in a state of incandescence by a current of lower electromotive force. Lane Fox and Edison, in 1878, proposed to employ platinum wires covered with films of lime, magnesia, steatite, or with the rarer oxides, zirconia, thoria, &c.; and Lane Fox, in 1879, suggested as an incandescent substance a mixture of particles of carbon with the earthy oxides. These earthy oxides—magnesia, lime and the oxides of the rare earths, such as thoria, zirconia, erbia, yttria, &c.—possess the peculiarity that at ordinary temperatures they are practically non-conductors, but at very high temperatures their resistance at a certain point rapidly falls, and they become fairly good conductors. Hence if they can once be brought into a state of incandescence a current can pass through them and maintain them in that state. But at this temperature they give up oxygen to carbon; hence no mixtures of earthy oxides with carbon are permanent when heated, and failure has attended all attempts to use a carbon filament covered with such substances as thoria, zirconia or other of the rare oxides.
H. W. Nernst in 1897, however, patented an incandescent lamp in which the incandescent body consists entirely of a slender rod or filament of magnesia. If such a rod is heated by the oxy-hydrogen blowpipe to a high Nernst lamp. temperature it becomes conductive, and can then be maintained in an intensely luminous condition by passing a current through it after the flame is withdrawn. Nernst found that by mixing together, in suitable proportions, oxides of the rare earths, he was able to prepare a material which can be formed into slender rods and threads, and which is rendered sufficiently conductive to pass a current with an electromotive force as low as 100 volts, merely by being heated for a few moments with a spirit lamp, or even by the radiation from a neighbouring platinum spiral brought to a state of incandescence.
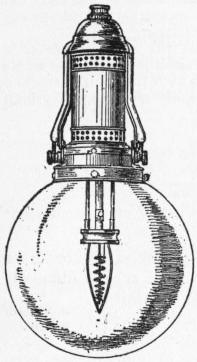 |
| Fig. 17.—Nernst Lamp A Type. |
 |
| Fig. 18.—Nernst Lamp, Burners for B Type. a, low voltage; b, high voltage. |
The Nernst lamp, therefore (fig. 17), consists of a slender rod of the mixed oxides attached to platinum wires by an oxide paste. Oxide filaments of this description are not enclosed in an exhausted glass vessel, and they can be brought, without risk of destruction, to a temperature considerably higher than a carbon filament; hence the lamp has a higher luminous efficiency. The material now used for the oxide rod or “glower” of Nernst lamps is a mixture of zirconia and yttria, made into a paste and squirted or pressed into slender rods. This material is non-conductive when cold, but when slightly heated it becomes conductive and then falls considerably in resistance. The glower, which is straight in some types of the lamp but curved in others, is generally about 3 or 4 cm. long and 1 or 2 mm. in diameter. It is held in suitable terminals, and close to it, or round it, but not touching it, is a loose coil of platinum wire, also covered with oxide and called the “heater” (fig. 18). In series with it is a spiral of iron wire, enclosed in a bulb full of hydrogen, which is called the “ballast resistance.” The socket also contains a switch controlled by an electromagnet. When the current is first switched on it passes through the heater coil which, becoming incandescent, by radiation heats the glower until it becomes conductive. The glower then takes current, becoming itself brilliantly incandescent, and the electromagnet becoming energized switches the heater coil out of circuit. The iron ballast wire increases in resistance with increase of current, and so operates to keep the total current through the glower constant in spite of small variations of circuit voltage. The disadvantages of the lamp are (1) that it does not light immediately after the current is switched on and is therefore not convenient for domestic use; (2) that it cannot be made in small light units such as 5 c.p.; (3) that the socket and fixture are large and more complicated than for the carbon filament lamp. But owing to the higher temperature, the light is whiter than that of the carbon glow lamp, and the efficiency or candle power per watt is greater. Since, however, the lamp must be included in an opal globe, some considerable part of this last advantage is lost. On the whole the lamp has found its field of operation rather in external than in domestic lighting.
Great efforts were made in the latter part of the 19th century and the first decade of the 20th to find a material for the filament of an incandescent lamp which could replace carbon and yet not require a preliminary heating like the Metallic filament lamps. oxide glowers. This resulted in the production of refractory metallic filament lamps made of osmium, tantalum, tungsten and other rare metals. Auer von Welsbach suggested the use of osmium. This metal cannot be drawn into wire on account of its brittleness, but it can be made into a filament by mixing the finely divided metal with an organic binding material which is carbonized in the usual way at a high temperature, the osmium particles then cohering. The difficulty has hitherto been to construct in this way metallic filament lamps of low candle power (16 c.p.) for 220 volt circuits, but this is being overcome. When used on modern supply circuits of 220 volts a number of lamps may be run in series, or a step-down transformer employed.
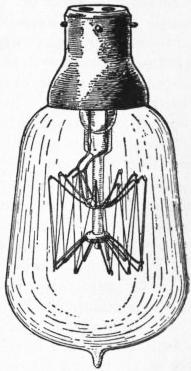 |
| Fig. 19.—Tantalum Lamp. |
The next great improvement came when W. von Bolton produced the tantalum lamp in 1904. There are certain metals known to have a melting point about 2000° C. or upwards, and of these tantalum is one. It can be produced from the potassium tantalo-fluoride in a pulverulent form. By carefully melting it in vacuo it can then be converted into the reguline form and drawn into wire. In this condition it has a density of 16.6 (water = 1), is harder than platinum and has greater tensile strength than steel, viz. 95 kilograms per sq. mm., the value for good steel being 70 to 80 kilograms per sq. mm. The electrical resistance at 15° C. is 0.146 ohms per metre with section of 1 sq. mm. after annealing at 1900° C. in vacuo and therefore about 6 times that of mercury; the temperature coefficient is 0.3 per degree C. At the temperature assumed in an incandescent lamp when working at 1.5 watts per c.p. the resistance is 0.830 ohms per metre with a section of 1 sq. mm. The specific heat is 0.0365. Bolton invented methods of producing tantalum in the form of a long fine wire 0.05 mm. in diameter. To make a 25 c.p. lamp 650 mm., or about 2 ft., of this wire are wound backwards and forwards zigzag on metallic supports carried on a glass frame, which is sealed into an exhausted glass bulb. The tantalum lamp so made (fig. 19), working on a 110 volt circuit takes 0.36 amperes or 39 watts, and hence has an efficiency of about 1.6 watts per c.p. The useful life, that is the time in which it loses 20% of its initial candle power, is about 400-500 hours, but in general a life of 800-1000 hours can be obtained. The bulb blackens little in use, but the life is said to be shorter with alternating than with direct current. When worked on alternating current circuits the filament after a time breaks up into sections which become curiously sheared with respect to each other but still maintain electrical contact. The resistance of tantalum increases with the temperature; hence the temperature coefficient is positive, and sudden rises in working voltage do not cause such variations in candle-power as in the case of the carbon lamp.
Patents have also been taken out for lamps made with filaments of such infusible metals as tungsten and molybdenum, and Siemens and Halske, Sanders and others, have protected methods for employing zirconium and other rare metals. According to the patents of Sanders (German patents Nos. 133701, 137568, 137569) zirconium filaments are manufactured from the hydrogen or nitrogen compounds of the rare earths by the aid of some organic binding material. H. Kuzel of Vienna (British Patent No. 28154 of 1904) described methods of making metallic filaments from any metal. He employs the metals in a colloidal condition, either as hydrosol, organosol, gel, or colloidal suspension. The metals are thus obtained in a gelatinous form, and can be squirted into filaments which are dried and reduced to the metallic form by passing an electric current through them (Electrician, 57, 894). This process has a wide field of application, and enables the most refractory and infusible metals to be obtained in a metallic wire form. The zirconium and tungsten wire lamps are equal to or surpass the tantalum lamp in efficiency and are capable of giving light, with a useful commercial life, at an efficiency of about one watt per candle. Lamps called osram lamps, with filaments composed of an alloy of osmium and tungsten (wolfram), can be used with a life of 1000 hours when run at an efficiency of about 1.5 watts per candle.
Tungsten lamps are made by the processes of Just and Hanaman (German patent No. 154262 of 1903) and of Kuzel, and at a useful life of 1000 hours, with a falling off in light-giving power of only 10-15%, they have been found to work at an efficiency of one to 1.25 watts per c.p. Further collected information on modern metallic wire lamps and the patent literature thereof will be found in an article in the Engineer for December 7, 1906.
Mention should also be made of the Helion filament glow lamp in which the glower is composed largely of silicon, a carbon filament being used as a base. This filament is said to have a number of interesting qualities and an efficiency of about 1 watt per candle (see the Electrician, 1907, 58, p. 567).
The mercury vapour lamps of P. Cooper-Hewitt, C. O. Bastian and others have a certain field of usefulness. If a glass tube, highly exhausted, contains mercury vapour and a mercury cathode and iron anode, a current can be Mercury vapour lamps. passed through it under high electromotive force and will then be maintained when the voltage is reduced. The mercury vapour is rendered incandescent and glows with a brilliant greenish light which is highly actinic, but practically monochromatic, and is therefore not suitable for general illumination because it does not reveal objects in their daylight colours. It is, however, an exceedingly economical source of light. A 3-ampere Cooper-Hewitt mercury lamp has an efficiency of 0.15 to 0.33 watts per candle, or practically the same as an arc lamp, and will burn for several thousand hours. A similar lamp with mercury vapour included in a tube of uviol glass specially transparent to ultra-violet light (prepared by Schott & Co. of Jena) seems likely to replace the Finsen arc lamp in the treatment of lupus. Many attempts have been made to render the mercury vapour lamp polychromatic by the use of amalgams of zinc, sodium and bismuth in place of pure mercury for the negative electrode.
An important matter in connexion with glow lamps is their photometry. The arrangement most suitable for the photometry and testing of incandescent lamps is a gallery or room large enough to be occupied by several workers, Photometry of glow lamps. the walls being painted dead black. The photometer, preferably one of the Lummer-Brodhun form, is set up on a gallery or bench. On one side of it must be fixed a working standard, which as first suggested by Fleming is preferably a large bulb incandescent lamp with a specially “aged” filament. Its candle-power can be compared, at regular intervals and known voltages, with that of some accepted flame standard, such as the 10 candle pentane lamp of Vernon Harcourt. In a lamp factory or electrical laboratory it is convenient to have a number of such large bulb standard lamps. This working standard should be maintained at a fixed distance on one side of the photometer, such that when worked at a standard voltage it creates an illumination of one candle-foot on one side of the photometer disk. The incandescent lamp to be examined is then placed on the other side of the photometer disk on a travelling carriage, so that it can be moved to and fro. Arrangements must be made to measure the current and the voltage of this lamp under test, and this is most accurately accomplished by employing a potentiometer (q.v.). The holder which carries the lamp should allow the lamp to be held with its axis in any required position; in making normal measurements the lamp should have its axis vertical, the filament being so situated that none of the turns or loops overlies another as seen from the photometer disk. Observations can then be made of the candle-power corresponding to different currents and voltages.
The candle-power of the lamp varies with the other variables in accordance with exponential laws of the following kind:—
If A is the current in amperes through the lamp, V the voltage or terminal potential difference, W the power absorbed in watts, c.p. the maximum candle-power, and a, b, c, &c., constants, it has been found that A and c.p. are connected by an exponential law such that
c.p. = aAx
For carbon filament lamps x is a number lying between 5 and 6, generally equal to 5.5 or 5.6. Also it has been found that c.p. = bW³ very nearly, and that
c.p. = cVy nearly
where c is some other constant, and for carbon filaments y is a number nearly equal to 6. It is obvious that if the candle-power of the lamp varies very nearly as the 6th power of the current and of the voltage, the candle-power must vary as the cube of the wattage.
Sir W. de W. Abney and E. R. Festing have also given a formula connecting candle-power and watts equivalent to c.p. = (W − d)² where d is a constant.
In the case of the tantalum lamp the exponent x has a value near to 6, but the exponent y is a number near to 4, and the same for the osmium filament. Hence for these metallic glowers a certain percentage variation of voltage does not create so great a variation in candle-power as in the case of the carbon lamp.
Curves delineating the relation of these variables for any incandescent lamp are called its characteristic-curves. The life or average duration is a function of W/c.p., or of the watts per candle-power, and therefore of the voltage at which the lamp is worked. It follows from the above relation that the watts per candle-power vary inversely as the fourth power of the voltage.
From limited observations it seems that the average life of a carbon-filament lamp varies as the fifth or sixth power of the watts per candle-power. If V is the voltage at which the lamp is worked and L is its average life, then L varies roughly as the twenty-fifth power of the reciprocal of the voltage, or
L = aV−25.
A closer approximation to experience is given by the formula
| log10L = 13.5 − | V | − | V² | . |
| 10 | 20,000 |
(See J. A. Fleming, “Characteristic Curves of Incandescent Lamps,” Phil. Mag. May 1885).
All forms of incandescent or glow lamps are found to deteriorate in light-giving power with use. In the case of carbon filaments this is due to two causes. As already explained, carbon is scattered from the filament and deposited Ageing of lamps. upon the glass, and changes also take place in the filament which cause it to become reduced in temperature, even when subjected to the same terminal voltage. In many lamps it is found that the first effect of running the lamp is slightly to increase its candle-power, even although the voltage be kept constant; this is the result of a small decrease in the resistance of the filament. The heating to which it is subjected slightly increases the density of the carbon at the outset; this has the effect of making the filament lower in resistance, and therefore it takes more current at a constant voltage. The greater part, however, of the subsequent decay in candle-power is due to the deposit of carbon upon the bulb, as shown by the fact that if the filament is taken out of the bulb and put into a new clean bulb the candle-power in the majority of cases returns to its original value. For every lamp there is a certain point in its career which may be called the “smashing-point,” when the candle-power falls below a certain percentage of the original value, and when it is advantageous to replace it by a new one. Variations of pressure in the electric supply exercise a prejudicial effect upon the light-giving qualities of incandescent lamps. If glow lamps, nominally of 100 volts, are supplied from a public lighting-station, in the mains of which the pressure varies between 90 and 110 volts, their life will be greatly abbreviated, and they will become blackened much sooner than would be the case if the pressure were perfectly constant. Since the candle-power of the lamp varies very nearly as the fifth or sixth power of the voltage, it follows that a variation of 10% in the electromotive force creates a variation of nearly 50% in the candle-power. Thus a 16 candle-power glow lamp, marked for use at 100 volts, was found on test to give the following candle-powers at voltages varying between 90 and 105: At 105 volts it gave 22.8 c.p.; at 100 volts, 16.7 c.p.; at 95 volts, 12.2 c.p.; and at 90 volts, 8.7 c.p. Thus a variation of 25% in the candle-power was caused by a variation in voltage of only 5%. The same kind of variation in working voltage exercises also a marked effect upon the average duration of the lamp. The following figures show the results of some tests on typical 3.1 watt lamps run at voltages above the normal, taking the average life when worked at the marked volts (namely, 100) as 1000 hours:
| At | 101 | volts the | life was | 818 | hours. |
| ” | 102 | ” | ” | 681 | ” |
| ” | 103 | ” | ” | 662 | ” |
| ” | 104 | ” | ” | 452 | ” |
| ” | 105 | ” | ” | 374 | ” |
| ” | 106 | ” | ” | 310 | ” |
Self-acting regulators have been devised by which the voltage at the points of consumption is kept constant, even although it varies at the point of generation. If, however, Voltage regulators. such a device is to be effective, it must operate very quickly, as even the momentary effect of increased pressure is felt by the lamp. It is only therefore where the working pressure can be kept exceedingly constant that high-efficiency lamps can be advantageously employed, otherwise the cost of lamp renewals more than counterbalances the economy in the cost of power. The slow changes that occur in the resistance of the filament make themselves evident by an increase in the watts per candle-power. The following table shows some typical figures indicating the results of ageing in a 16 candle-power carbon-filament glow lamp:—
| Hours run. | Candle-Power. | Watts per Candle-Power. |
| 0 | 16.0 | 3.16 |
| 100 | 15.8 | 3.26 |
| 200 | 15.86 | 3.13 |
| 300 | 15.68 | 3.37 |
| 400 | 15.41 | 3.53 |
| 500 | 15.17 | 3.51 |
| 600 | 14.96 | 3.54 |
| 700 | 14.74 | 3.74 |
The gradual increase in watts per candle-power shown by this table does not imply necessarily an increase in the total power taken by the lamp, but is the consequence of the decay in candle-power produced by the blackening of the lamp. Therefore, to estimate the value of an incandescent lamp the user must take into account not merely the price of the lamp and the initial watts per candle-power, but the rate of decay of the lamp.
The scattering of carbon from the filament to the glass bulb produces interesting physical effects, which have been studied by T. A. Edison, W. H. Preece and J. A. Fleming. If into an ordinary carbon-filament glow lamp a Edison effect. platinum plate is sealed, not connected to the filament but attached to a third terminal, then it is found that when the lamp is worked with continuous current a galvanometer connected in between the middle plate and the positive terminal of the lamp indicates a current, but not when connected in between the negative terminal of the lamp and the middle plate. If the middle plate is placed between the legs of a horse-shoe-shaped filament, it becomes blackened most quickly on the side facing the negative leg. This effect, commonly called the Edison effect, is connected with an electric discharge and convection of carbon which takes place between the two extreme ends of the filament, and, as experiment seems to show, consists in the conveyance of an electric charge, either by carbon molecules or by bodies smaller than molecules. There is, however, an electric discharge between the ends of the filament, which rapidly increases with the temperature of the filament and the terminal voltage; hence one of the difficulties of manufacturing high-voltage glow lamps, that is to say, glow lamps for use on circuits having an electromotive force of 200 volts and upwards, is the discharge from one leg of the filament to the other.
A brief allusion may be made to the mode of use of incandescent lamps for interior and private lighting. At the present time hardly any other method of distribution is adopted than that of an arrangement in parallel; that is Domestic use. to say, each lamp on the circuit has one terminal connected to a wire which finally terminates at one pole of the generator, and its other terminal connected to a wire leading to the other pole. The lamp filaments are thus arranged between the conductors like the rungs of a ladder. In series with each lamp is placed a switch and a fuse or cut-out. The lamps themselves are attached to some variety of ornamental fitting, or in many cases suspended by a simple pendant, consisting of an insulated double flexible wire attached at its upper end to a ceiling rose, and carrying at the lower end a shade and socket in which the lamp is placed. Lamps thus hung head downwards are disadvantageously used because their end-on candle-power is not generally more than 60% of their maximum candle-power. In interior lighting one of the great objects to be attained is uniformity of illumination with avoidance of harsh shadows. This can only be achieved by a proper distribution of the lamps. It is impossible to give any hard and fast rules as to what number must be employed in the illumination of any room, as a great deal depends upon the nature of the reflecting surfaces, such as the walls, ceilings, &c. As a rough guide, it may be stated that for every 100 sq. ft. of floor surface one 16 candle-power lamp placed about 8 ft. above the floor will give a dull illumination, two will give a good illumination and four will give a brilliant illumination. We generally judge of the nature of the illumination in a room by our ability to read comfortably in any position. That this may be done, the horizontal illumination on the book should not be less than one candle-foot. The following table shows approximately the illuminations in candle-feet, in various situations, derived from actual experiments:—
| In a well-lighted room on the floor or tables | 1.0 to 3.0 c.f. |
| On a theatre stage | 3.0 to 4.0 c.f. |
| On a railway platform | .05 to .5 c.f. |
| In a picture gallery | .65 to 3.5 c.f. |
| The mean daylight in May in the interior of a room | 30.0 to 40.0 c.f. |
| In full sunlight | 7000 to 10,000 c.f. |
| In full moonlight | 1/60th to 1/100th c.f. |
From an artistic point of view, one of the worst methods of lighting a room is by pendant lamps, collected in single centres in large numbers. The lights ought to be distributed in different portions of the room, and so shaded that the light is received only by reflection from surrounding objects. Ornamental effects are frequently produced by means of candle lamps in which a small incandescent lamp, imitating the flame of a candle, is placed upon a white porcelain tube as a holder, and these small units are distributed and arranged in electroliers and brackets. For details as to the various modes of placing conducting wires in houses, and the various precautions for safe usage, the reader is referred to the article Electricity Supply. In the case of low voltage metallic filament lamps when the supply is by alternating current there is no difficulty in reducing the service voltage to any lower value by means of a transformer. In the case of direct current the only method available for working such low voltage lamps off higher supply voltages is to arrange the lamps in series.
Additional information on the subjects treated above may be found in the following books and original papers:—
Mrs Ayrton, The Electric Arc (London, 1900); Houston and Kennelly, Electric Arc Lighting and Electric Incandescent Lighting; S. P. Thompson, The Arc Light, Cantor Lectures, Society of Arts (1895); H. Nakano, “The Efficiency of the Arc Lamp,” Proc. American Inst. Elec. Eng. (1889); A. Blondel, “Public and Street Lighting by Arc Lamps,” Electrician, vols. xxxv. and xxxvi. (1895); T. Heskett, “Notes on the Electric Arc,” Electrician, vol. xxxix. (1897); G. S. Ram, The Incandescent Lamp and its Manufacture (London, 1895); J. A. Fleming, Electric Lamps and Electric Lighting (London, 1899); J. A. Fleming, “The Photometry of Electric Lamps,” Jour. Inst. Elec. Eng. (1903), 32, p. 1 (in this paper a copious bibliography of the subject of photometry is given); J. Dredge, Electric Illumination (2 vols., London, 1882, 1885); A. P. Trotter, “The Distribution and Measurement of Illumination,” Proc. Inst. C.E. vol. cx. (1892); E. L. Nichols, “The Efficiency of Methods of Artificial Illumination,” Trans. American Inst. Elec. Eng. vol. vi. (1889); Sir W. de W. Abney, Photometry, Cantor Lectures, Society of Arts (1894); A. Blondel, “Photometric Magnitudes and Units,” Electrician (1894); J. E. Petavel, “An Experimental Research on some Standards of Light,” Proc. Roy. Soc. lxv. 469 (1899); F. Jehl, Carbon-Making for all Electrical Purposes (London, 1906); G. B. Dyke, “On the Practical Determination of the Mean Spherical Candle Power of Incandescent and Arc Lamps,” Phil. Mag. (1905); the Preliminary Report of the Sub-Committee of the American Institute of Electrical Engineers on “Standards of Light”; Clifford C. Paterson, “Investigations on Light Standards and the Present Condition of the High Voltage Glow Lamp,” Jour. Inst. Elec. Eng. (January 24, 1907); J. Swinburne, “New Incandescent Lamps,” Jour. Inst. Elec. Eng. (1907); L. Andrews, “Long Flame Arc Lamps,” Jour. Inst. Elec. Eng. (1906); W. von Bolton and O. Feuerlein, “The Tantalum Lamp,” The Electrician (Jan. 27, 1905). Also the current issues of The Illuminating Engineer.
Commercial Aspects.—The cost of supplying electricity depends more upon the rate of supply than upon the quantity supplied; or, as John Hopkinson put it, “the cost of supplying electricity for 1000 lamps for ten hours is very much Methods of charging. less than ten times the cost of supplying the same number of lamps for one hour.” Efforts have therefore been made to devise a system of charge which shall in each case bear some relation to the cost of the service. Consumers vary largely both in respect to the quantity and to the period of their demands, but the cost of supplying any one of them with a given amount of electricity is chiefly governed by the amount of his maximum demand at any one time. The reason for this is that it is not generally found expedient to store electricity in large quantities. Electricity supply works generate the electricity for the most part at the moment it is used by the consumer. Electric lamps are normally in use on an average for only about four hours per day, and therefore the plant and organization, if employed for a lighting load only, are idle and unremunerative for about 20 hours out of the 24. It is necessary to have in readiness machinery capable of supplying the maximum possible requirements of all the consumers at any hour, and this accounts for a very large proportion of the total cost. The cost of raw material, viz. coal, water and stores consumed in the generation of electricity sold, forms relatively only a small part of the total cost, the major part of which is made up of the fixed charges attributable to the time during which the works are unproductive. This makes it very desirable to secure demands possessing high “load” and “diversity” factors. The correct way to charge for electricity is to give liberal rebates to those consumers who make prolonged and regular use of the plant, that is to say, the lower the “peak” demand and the more continuous the consumption, the better should be the discount. The consumer must be discouraged from making sudden large demands on the plant, and must be encouraged, while not reducing his total consumption, to spread his use of the plant over a large number of hours during the year. Mr Arthur Wright has devised a tariff which gives effect to this principle. The system necessitates the use of a special indicator—not to measure the quantity of electricity consumed, which is done by the ordinary meter—but to show the maximum amount of current taken by the consumer at any one time during the period for which he is to be charged. In effect it shows the proportion of plant which has had to be kept on hand for his use. If the indicator shows that say twenty lamps is the greatest number which the consumer has turned on simultaneously, then he gets a large discount on all the current which his ordinary meter shows that he has taken beyond the equivalent of one hour’s daily use of those twenty lamps. Generally the rate charged under this system is 7d. per unit for the equivalent of one hour’s daily use of the maximum demand and 1d. per unit for all surplus. It is on this principle that it pays to supply current for tramway and other purposes at a price which primâ facie is below the cost of production; it is only apparently so in comparison with the cost of producing electricity for lighting purposes. In the case of tramways the electricity is required for 15 or 16 hours per day. Electricity for a single lamp would cost on the basis of this “maximum-demand-indicator” system for 15 hours per day only 1.86d. per unit. In some cases a system of further discounts to very large consumers is combined with the Wright system. Some undertakers have abandoned the Wright system in favour of average flat rates, but this does not imply any failure of the Wright system; on the contrary, the system, having served to establish the most economical consumption of electricity, has demonstrated the average rate at which the undertakers are able to give the supply at a fair profit, and the proportion of possible new customers being small the undertakers find it a simplification to dispense with the maximum demand indicator. But in some cases a mistake has been made by offering the unprofitable early-closing consumers the option of obtaining electricity at a flat rate much lower than their load-factor would warrant and below cost price. The effect of this is to nullify the Wright system of charging, for a consumer will not elect to pay for his electricity on the Wright system if he can obtain a lower rate by means of a flat rate system. Thus the long-hour profitable consumer is made to pay a much higher price than he need be charged, in order that the unprofitable short-hour consumer may be retained and be made actually still more unprofitable. It is not improbable that ultimately the supply will be charged for on the basis of a rate determined by the size and character of the consumer’s premises, or the number and dimensions of the electrical points, much in the same way as water is charged for by a water rate determined by the rent of the consumer’s house and the number of water taps.
Most new houses within an electricity supply area are wired for electricity during construction, but in several towns means have to be taken to encourage small shopkeepers and tenants of small houses to use electricity by removing Wiring of houses. the obstacle of the first outlay on wiring. The cost of wiring may be taken at 15s. to £2 per lamp installed including all necessary wire, switches, fuses, lamps, holders, casing, but not electroliers or shades. Many undertakers carry out wiring on the easy payment or hire-purchase system. Parliament has sanctioned the adoption of these systems by some local authorities and even authorized them to do the work by direct employment of labour. The usual arrangement is to make an additional charge of ½d. per unit on all current used, with a minimum payment of 1s. per 8 c.p. lamp, consumers having the option of purchasing the installation at any time on specified conditions. The consumer has to enter into an agreement, and if he is only a tenant the landlord has to sign a memorandum to the effect that the wiring and fittings belong to the supply undertakers. Several undertakers have adopted a system of maintenance and renewal of lamps, and at least one local authority undertakes to supply consumers with lamps free of charge.
There is still considerable scope for increasing the business of electricity supply by judicious advertising and other methods. Comparisons of the kilowatt hour consumption per capita in various towns show that where an energetic Consumption. policy has been pursued the profits have improved by reason of additional output combined with increased load factor. The average number of equivalent 8 c.p. lamps connected per capita in the average of English towns is about 1.2. The average number of units consumed per capita per annum is about 23, and the average income per capita per annum is about 5s. In a number of American cities 20s. per capita per annum is obtained. In the United States a co-operative electrical development association canvasses both the general public and the electricity supply undertakers. Funds are provided by the manufacturing companies acting in concert with the supply authorities and contractors, and the spirit underlying the work is to advertise the merits of electricity—not any particular company or interest. Their efforts are directed to securing new consumers and stimulating the increased and more varied use of electricity among actual consumers.
All supply undertakers are anxious to develop the consumption of electricity for power purposes even more than for lighting, but the first cost of installing electric motors is a deterrent to the adoption of electricity in small factories and shops, and most undertakers are therefore prepared to let out motors, &c., on hire or purchase on varying terms according to circumstances.
A board of trade unit will supply one 8 c.p. carbon lamp of 30 hours or 30 such lamps for one hour. In average use an incandescent lamp will last about 800 hours, which is equal to about 12 months normal use; a good lamp will frequently last more than double this time before it breaks down.
A large number of towns have adopted electricity for street lighting. Frank Bailey has furnished particulars of photometric tests which he has made on new and old street lamps in the city of London. From these tests the following comparative figures are deduced:—
| Average total Cost per c.p. per annum. |
|
| Gas— | |
| Double burner ordinary low pressure incandescent (mean of six tests) | 11.1d. |
| Single burner high-pressure gas | 9.0 |
| Double burner high-pressure gas | 11.7 |
| Arc lamp— | |
| Old type of lantern | 8 |
| Flame arc | 5 |
From these tests of candle-power the illumination at a distance of 100 ft. from the source is estimated as follows:—
| Candle Ft. | Ratio. | ||
| Double ordinary incandescent gas lamp illumination | 0.013 | = | 1.0 |
| Single high pressure ordinary incandescent gas lamp illumination | 0.016 | = | 1.24 |
| Double high pressure ordinary incandescent gas lamp illumination | 0.027 | = | 2.10 |
| Ordinary arc lamp | 0.060 | = | 4.50 |
| Flame arc lamp | 0.120 | = | 9.00 |
The cost of electricity, light for light, is very much less than that of gas. The following comparative figures relating to street lighting at Croydon have been issued by the lighting committee of that corporation:—
| Type of Lamp. | Number of Lamps. |
Distance apart (yds.) |
Total Cost. |
Average c.p. per Mile. |
Cost per c.p. per annum. |
| Incandescent gas | 2,137 | 80 | £7,062 | 839 | 15.86d. |
| Incandescent electric | 90 | 66 | 288 | 1,373 | 13.71 |
| Electric arcs | 428 | 65 | 7,212 | 10,537 | 11.32 |
Apart from cheaper methods of generation there are two main sources of economy in electric lighting. One is the improved arrangement and use of electrical installations, and the other is the employment of lamps of higher efficiency. As regards the first, increased attention has been given to the position, candle-power and shading of electric lamps so as to give the most effective illumination in varying circumstances and to avoid excess of light. The ease with which electric lamps may be switched on and off from a distance has lent itself to arrangements whereby current may be saved by switching off lights not in use and by controlling the number of lamps required to be alight at one time on an electrolier. Appreciable economies are brought about by the scientific disposition of lights and the avoidance of waste in use. As regards the other source of economy, the Nernst, the tantalum, the osram, and the metallized carbon filament lamp, although costing more in the first instance than carbon lamps, have become popular owing to their economy in current consumption. Where adopted largely they have had a distinct effect in reducing the rate of increase of output from supply undertakings, but their use has been generally encouraged as tending towards the greater popularity of electric light and an ultimately wider demand. Mercury vapour lamps for indoor and outdoor lighting have also proved their high efficiency, and the use of flame arc lamps has greatly increased the cheapness of outdoor electric lighting.
The existence of a “daylight load” tends to reduce the all-round cost of generating and distributing electricity. This daylight load is partly supplied by power for industrial purposes and partly by the demand for electricity in many domestic operations. The use of electric heating and cooking apparatus (including radiators, ovens, grills, chafing dishes, hot plates, kettles, flat-irons, curling irons, &c.) has greatly developed, and provides a load which extends intermittently throughout the greater part of the twenty-four hours. Electric fans for home ventilation are also used, and in the domestic operations where a small amount of power is required (as in driving sewing machines, boot cleaners, washing machines, mangles, knife cleaners, “vacuum” cleaners, &c.) the electric motor is being largely adopted. The trend of affairs points to a time when the total demand from such domestic sources will greatly exceed the demand for lighting only. The usual charges for current to be used in domestic heating or power operations vary from 1d. to 2d. per unit. As the demand increases the charges will undergo reduction, and there will also be a reflex action in bringing down the cost of electricity for lighting owing to the improved load factor resulting from an increase in the day demand. In the cooking and heating and motor departments also there has been improvement in the efficiency of the apparatus, and its economy is enhanced by the fact that current may be switched on and off as required.
The Board of Trade are now prepared to receive electric measuring instruments for examination or testing at their Testing meters. electrical standardizing laboratory, where they have a battery power admitting of a maximum current of 7000 amperes to be dealt with. The London county council and some other corporations are prepared upon requisition to appoint inspectors to test meters on consumers’ premises.
All supply undertakers now issue rules and regulations for the efficient wiring of electric installations. The rules and regulations issued by the institution of electrical engineers have been accepted by many local authorities and companies, and Wiring rules. also by many of the fire insurance companies. The Phoenix fire office rules were the first to be drawn up, and are adopted by many of the fire offices, but some other leading insurance offices have their own rules under which risks are accepted without extra premium. In the opinion of the insurance companies “the electric light is the safest of all illuminants and is preferable to any others when the installation has been thoroughly well put up.” Regulations have also been issued by the London county council in regard to theatres, &c., by the national board of fire underwriters of America (known as the “National Electrical Code”), by the fire underwriters association of Victoria (Commonwealth of Australia), by the Calcutta fire insurance agents association and under the Canadian Electric Light Inspection Act. In Germany rules have been issued by the Verband Deutscher Elektrotechniker and by the union of private fire insurance companies of Germany, in Switzerland by the Association Suisse des électriciens, in Austria by the Elektrotechnischer Verein of Vienna, in France by ministerial decree and by the syndicat professionel des industries électriques. (For reprints of these regulations see Electrical Trades Directory.)
1 Journ. Inst. Elec. Eng. 28, p. 1. The authors of this paper give numerous instructive curves taken with the oscillograph, showing the form of the arc P.D. and current curves for a great variety of alternating-current arcs.


