Gastropoda, the second of the five classes of animals constituting the phylum Mollusca. For a discussion of the relationship of the Gastropoda to the remaining classes of the phylum, see Mollusca.
The Gastropoda are mainly characterized by a loss of symmetry, produced by torsion of the visceral sac. This torsion may be resolved into two successive movements. The first is a ventral flexure in the antero-posterior or sagittal plane; the result of this is to approximate the two ends of the alimentary canal. In development, the openings of the mantle-cavity and the anus are always originally posterior; later they are brought forward ventrally. During this first movement flexure is also produced by the coiling of the visceral sac and shell; primitively the latter was bowl-shaped; but the ventral flexure, which brings together the two extremities of the digestive tube, gives the visceral sac the outline of a more or less acute cone. The shell necessarily takes this form also, and then becomes coiled in a dorsal or anterior plane—that is to say, it becomes exogastric. This condition may be seen in embryonic Patellidae, Fissurellidae and Trochidae (fig. 1, A), and agrees with the method of coiling of a mollusc without lateral torsion, such as Nautilus. But ultimately the coil becomes ventral or endogastric, in consequence of the second torsion movement then apparent.
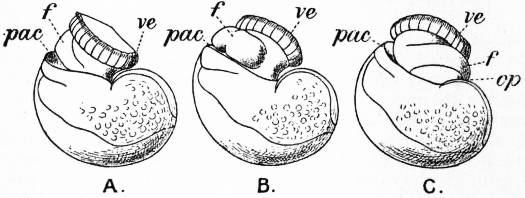 |
|
| From Lankester’s Treatise on Zoology. | |
| Fig. 1.—Three stages in the development of Trochus, during the process of torsion. (After Robert.) | |
A, Nearly symmetrical larva (veliger). B, A stage 1½ hours later than A. C, A stage 3½ hours later than B. f, Foot. |
op, Operculum. pac, Pallial cavity. ve, Velum. |
The shell is represented as fixed, while the head and foot rotate from left to right. In reality the head and foot are fixed and the shell rotates from right to left.
The second movement is a lateral torsion of the visceral mass, the foot remaining a fixed point; this torsion occurs in a plane approximately at right angles to that of the first movement, and carries the pallial aperture and the anus from behind forwards. If, at this moment, the animal were placed with mouth and ventral surface turned towards the observer, this torsion carries the circumanal complex in a clockwise direction (along the right side in dextral forms) through 180° as compared with its primitive condition. The (primitively) right-hand organs of the complex thus become left-hand, and vice versa. The visceral commissure, while still surrounding the digestive tract, becomes looped; its right half, with its proper ganglion, passes to the left side over the dorsal face of the alimentary canal (whence the name supra-intestinal), while the left half passes below towards the right side, thus originating the name infra-intestinal given to this half and to its ganglion. Next, the shell, the coil of which was at first exogastric, being also included in this rotation through 180°, exhibits an endogastric coiling (fig. 1, B, C). This, however, is not generally retained in one plane, and the spire projects, little by little, on the side which was originally left, but finally becomes right (in dextral forms, with a clockwise direction, if viewed from the side of the spire; but counter-clockwise in sinistral forms). Finally, the original symmetry of the circumanal complex vanishes; the anus leaves the centre of the pallial cavity and passes towards the right side (left side in sinistral forms); the organs of this side become atrophied and disappear. The essential feature of the asymmetry of Gastropoda is the atrophy or disappearance of the primitively left half of the circumanal complex (the right half in sinistral forms), including the gill, the auricle, the osphradium, the hypobranchial gland and the kidney.
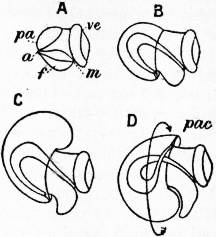 |
| From Lankester’s Treatise on Zoology. |
| Fig. 2.—Four stages in the development of a Gastropod showing the process of body torsion. (After Robert.) |
A, Embryo without flexure. B, Embryo with ventral flexure of the intestine. C, Embryo with ventral flexure and exogastric shell. D, Embryo with lateral torsion and an endogastric shell. a, Anus. f, Foot. m, Mouth. pa, Mantle. pac, Pallial cavity. ve, Velum. |
In dextral Gastropods the only structure found on the topographically right side of the rectum is the genital duct. But this is not part of the primitive complex. It is absent in the most primitive and symmetrical forms, such as Haliotis and Pleurotomaria. Originally the gonads opened into the kidneys. In the most primitive existing Gastropods the gonad opens into the right kidney (Patellidae, Trochidae, Fissurellidae). The gonaduct, therefore, is derived from the topographically right kidney. The transformation has been actually shown to take place in the development of Paludina. In a dextral Gastropod the shell is coiled in a right-handed spiral from apex to mouth, and the spiral also projects to the right of the median plane of the animal.
When the shell is sinistral the asymmetry of the organs is usually reversed, and there is a complete situs inversus viscerum, the direction of the spiral of the shell corresponding to the position of the organs of the body. Triforis, Physa, Clausilia are examples of sinistral Gastropods, but reversal also occurs as an individual variation among forms normally dextral. But there are forms in which the involution is “hyperstrophic,” that is to say, the turns of the spire projecting but slightly, the spire, after flattening out gradually, finally becomes re-entrant and transformed into a false umbilicus; at the same time that part which corresponds to the umbilicus of forms with a normal coil projects and constitutes a false spire; the coil thus appears to be sinistral, although the asymmetry remains dextral, and the coil of the operculum (always the opposite to that of the shell) sinistral (e.g. Lanistes among Streptoneura, Limacinidae among Opisthobranchia). The same, mutatis mutandis, may occur in sinistral shells.
The problem of the causes of the torsion of the Gastropod body has been much discussed. E.R. Lankester in the ninth edition of this work attributed it to the pressure of the shell and visceral hump towards the right side. He referred also to the nautiloid shell of the larva falling to one side. But these are two distinct processes. In the larva a nautiloid shell is developed which is coiled exogastrically, that is, dorsally, and the pallial cavity is posterior or ventral (fig. 2, C): the larva therefore resembles Nautilus in the relations of body and shell. The shell then rotates towards the left side through 180°, so that it becomes ventral or endogastric (fig. 2, D). The pallial cavity, with its organs, is by this torsion moved up the right side of the larva to the dorsal surface, and thus the left organs become right and vice versa. In the subsequent growth of the shell the spire comes to project on the right side, which was originally the left. Neither the rotation of the shell as a whole nor its helicoid spiral coiling is the immediate cause of the torsion of the body in the individual, for the direction of the torsion is indicated in the segmentation of the ovum, in which there is a complete reversal of the cleavage planes in sinistral as compared with dextral forms. The facts, however, strongly suggest that the original cause of the torsion was the weight of the exogastric shell and visceral hump, which in an animal creeping on its ventral surface necessarily fell over to one side. It is not certain that the projection of the spire to the originally left side of the shell has anything to do with the falling over of the shell to that side. The facts do not support such a suggestion. In the larva there is no projection at the time the torsion takes place. In some forms the coiling disappears in the adult, leaving the shell simply conical as in Patellidae, Fissurellidae, &c., and in some cases the shell is coiled in one plane, e.g. Planorbis. In all these cases the torsion and asymmetry of the body are unaffected.
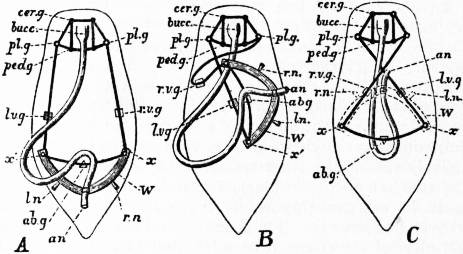 |
|
| Fig 3.—Sketch of a model designed so as to show the effect of torsion or rotation of the visceral hump in Streptoneurous Gastropoda. | |
A, Unrotated ancestral condition. B, Quarter-rotation. C, Complete semi-rotation (the limit). an, Anus. ln, rn, Primarily left nephridium and primarily right nephridium. lvg, Primarily left (subsequently the sub-intestinal) visceral ganglion. rvg, Primarily right (subsequently the sub-intestinal) visceral ganglion. |
cerg, Cerebral ganglion. plg, Pleural ganglion. pedg, Pedal ganglion. abg, Abdominal ganglion. bucc, Buccal mass. W, Wooden arc representing the base-line of the wall of the visceral hump. x, x′, Pins fastening the elastic cord (representing the visceral nerve loop) to W. |
The characteristic torsion attains its maximum effect among the majority of the Streptoneura. It is followed in some specialized Heteropoda and in the Euthyneura by a torsion in the opposite direction, or detorsion, which brings the anus farther back and untwists the visceral commissure (see Euthyneura, below). This conclusion has shown that the Euthyneura do not represent an archaic form of Gastropoda, but are themselves derived from streptoneurous forms. The difference between the two sub-classes has been shown to be slight; certain of the more archaic Tectibranchia (Actaeon) and Pulmonata (Chilina) still have the visceral commissure long and not untwisted. The fact that all the Euthyneura are hermaphrodite is not a fundamental difference; several Streptoneura are so, likewise Valvata, Oncidiopsis, Marsenina, Odostomia, Bathysciadium, Entoconcha.
Classification.—The class Gastropoda is subdivided as follows:
| Sub-class I. Streptoneura. | |
| Order 1. Aspidobranchia. | |
| Sub-order | 1. Docoglossa. |
| ” | 2. Rhipidoglossa. |
| Order 2. Pectinibranchia. | |
| Sub-order | 1. Taenioglossa. |
| Tribe | 1. Platypoda. |
| ” | 2. Heteropoda. |
| Sub-order | 2. Stenoglossa. |
| Tribe | 1. Rachiglossa. |
| ” | 2. Toxiglossa. |
| Sub-class II. Euthyneura. | |
| Order 1. Opisthobranchia. | |
| Sub-order | 1. Tectibranchia. |
| Tribe | 1. Bullomorpha. |
| ” | 2. Aplysiomorpha. |
| ” | 3. Pleurobranchomorpha. |
| Sub-order | 2. Nudibranchia. |
| Tribe | 1. Tritoniomorpha. |
| ” | 2. Doridomorpha. |
| ” | 3. Eolidomorpha. |
| ” | 4. Elysiomorpha. |
| Order 2. Pulmonata. | |
| Sub-order | 1. Basommatophora. |
| ” | 2. Stylommatophora. |
| Tribe | 1. Holognatha. |
| ” | 2. Agnatha. |
| ” | 3. Elasmognatha. |
| ” | 4. Ditremata. |
Sub-Class I.—Streptoneura
In this division the torsion of the visceral mass and visceral commissure is at its maximum, the latter being twisted into a figure of eight. The right half of the commissure with its ganglion is supra-intestinal, the left half with its ganglion infra-intestinal. In some cases each pleural ganglion is connected with the opposite branch of the visceral commissure by anastomosis with the pallial nerve, a condition which is called dialyneury; or there may be a direct connective from the pleural ganglion to the visceral ganglion of the opposite side, which is called zygoneury. The head bears only one pair of tentacles. The radular teeth are of several different kinds in each transverse row. The heart is usually posterior to the branchia (proso-branchiate). The sexes are usually separate.
The old division into Zygobranchia and Azygobranchia must be abandoned, for the Azygobranchiate Rhipidoglossa have much greater affinity to the Zygobranchiate Haliotidae and Fissurellidae than to the Azygobranchia in general. This is shown by the labial commissure and pedal cords of the nervous system, by the opening of the gonad into the right kidney, and by other points. Further, the Pleurotomariidae have been discovered to possess two branchiae. The sub-class is now divided into two orders: the Aspidobranchia in which the branchia or ctenidium is bipectinate and attached only at its base, and the Pectinibranchia in which the ctenidium is monopectinate and attached to the mantle throughout its length.
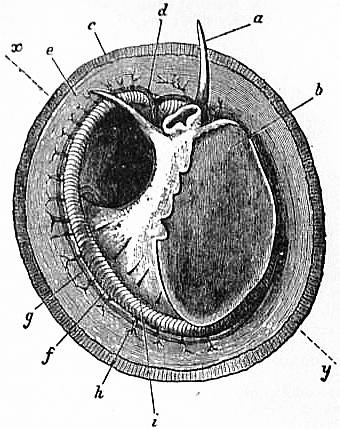 |
|
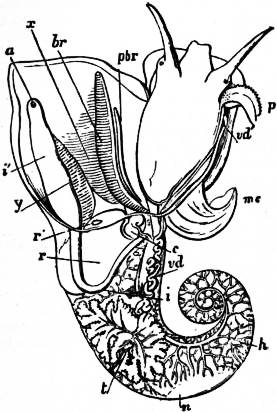
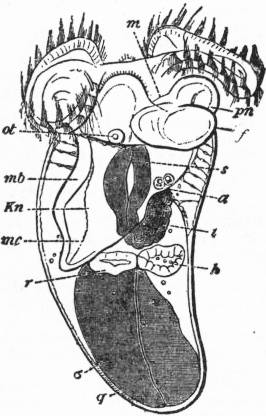
| Fig. 4.—The Common Limpet (Patella vulgata) in its shell, seen from the pedal surface. (Lankester.) | |
x, y, The median antero-posterior axis. a, Cephalic tentacle. b, Plantar surface of the foot. c, Free edge of the shell. d, The branchial efferent vessel carrying aerated blood to the auricle, and here interrupting the circlet of gill lamellae. |
e, Margin of the mantle-skirt. f, Gill lamellae (not ctenidia, but special pallial growths, comparable with those of Pleurophyllidia). g, The branchial efferent vessel. h, Factor of the branchial advehent vessel. i, Interspaces between the muscular bundles of the root of the foot, causing the separate areae seen in fig. 5, c. |
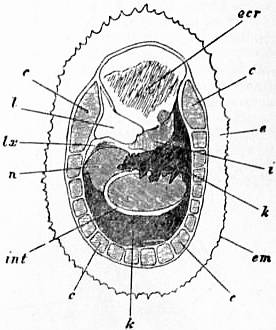 |
| Fig. 5.—Dorsal surface of the Limpet removed from its shell and deprived of its black pigmented epithelium; the internal organs are seen through the transparent body-wall. (Lankester.) |
c, Muscular bundles forming the root of the foot, and adherent to the shell. e, Free mantle-skirt. em, Tentaculiferous margin of the same. i, Smaller (left) nephridium. k, Larger (right) nephridium. l, Pericardium. lx, Fibrous septum, behind the pericardium. n, Liver. int, Intestine. ecr, Anterior area of the mantle-skirt over-hanging the head (cephalic hood). |
Order I. Aspidobranchia.—These are the most primitive Gastropods, retaining to a great degree the original symmetry of the organs of the pallial complex, having two kidneys, in some cases two branchiae, and two auricles. The gonad has no accessory organs and except in Neritidae no duct, but discharges into the right kidney.
Forms adapted to terrestrial life and to aerial respiration occur in various divisions of Gastropods, and do not constitute a single homogeneous group. Thus the Helicinidae, which are terrestrial, are now placed among the Aspidobranchia. In these there are neither branchia nor osphradium, and the pallial chamber which retains its large opening serves as a lung. Degeneration of the shell occurs in some members of the order. It is largely covered by the mantle in some Fissurellidae, is entirely internal in Pupilia and absent in Titiscaniidae.
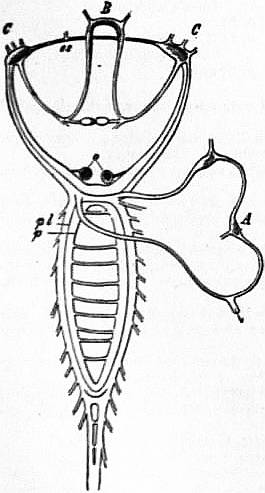
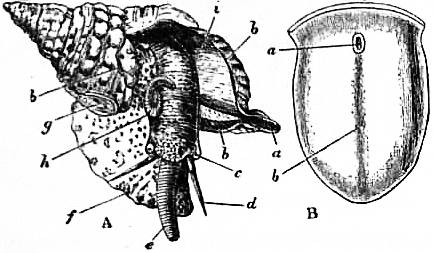
The common limpet is a specially interesting and abundant example of the more primitive Aspidobranchia. The foot of the limpet is a nearly circular disk of muscular tissue; in front, projecting from and raised above it, are the head and neck (figs. 4, 13). The visceral hump forms a low conical dome above the sub-circular foot, and standing out all round the base of this dome so as completely to overlap the head and foot, is the circular mantle-skirt. The depth of free mantle-skirt is greatest in front, where the head and neck are covered in by it. Upon the surface of the visceral dome, and extending to the edge of the free mantle-skirt, is the conical shell. When the shell is taken away (best effected by immersion in hot water) the surface of the visceral dome is found to be covered by a black-coloured epithelium, which may be removed, enabling the observer to note the position of some organs lying below the transparent integument (fig. 5). The muscular columns (c) attaching the foot to the shell form a ring incomplete in front, external to which is the free mantle-skirt. The limits of the large area formed by the flap over the head and neck (ecr) can be traced, and we note the anal papilla showing through and opening on the right shoulder, so to speak, of the animal into the large anterior region of the sub-pallial space. Close to this the small renal organ (i, mediad) and the larger renal organ (k, to the right and posteriorly) are seen, also the pericardium (l) and a coil of the intestine (int) embedded in the compact liver.
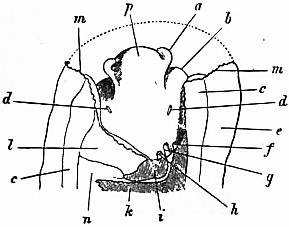 |
|
| Fig. 6.—Anterior portion of the same Limpet, with the overhanging cephalic hood removed. (Lankester.) | |
a, Cephalic tentacle. b, Foot. c, Muscular substance forming the root of the foot. d, The capito-pedal organs of Lankester (= rudimentary ctenidia). e, Mantle-skirt. f, Papilla of the larger nephridium. g, Anus. |
h, Papilla of the smaller nephridium. i, Smaller nephridium. k, Larger nephridium. l, Pericardium. m, Cut edge of the mantle-skirt. n, Liver. p, Snout. |
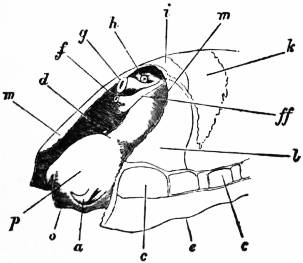 |
| Fig. 7.—The same specimen viewed from the left front, so as to show the sub-anal tract (ff) of the larger nephridium, by which it communicates with the pericardium. o, Mouth; other letters as in fig. 6. |
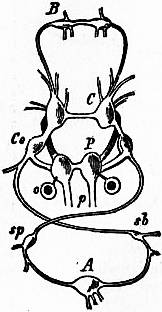
On cutting away the anterior part of the mantle-skirt so as to expose the sub-pallial chamber in the region of the neck, we find the right and left renal papillae (discovered by Lankester in 1867) on either side of the anal papilla (fig. 6), but no gills. If a similar examination be made of the allied genus Fissurella (fig. 17, d), we find right and left of the two renal apertures a right and left gill-plume or ctenidium, which here as in Haliotis and Pleurotomaria retain their original paired condition. In Patella no such plumes exist, but right and left of the neck are seen a pair of minute oblong yellow bodies (fig. 6, d), which were originally described by Lankester as orifices possibly connected with the evacuation of the generative products. On account of their position they were termed by him the “capito-pedal orifices,” being placed near the junction of head and foot. J.W. Spengel has, however, in a most ingenious way shown that these bodies are the representatives of the typical pair of ctenidia, here reduced to a mere rudiment. Near to each rudimentary ctenidium Spengel has discovered an olfactory patch or osphradium (consisting of modified epithelium) and an olfactory nerve-ganglion (fig. 8). It will be remembered that, according to Spengel, the osphradium of mollusca is definitely and intimately related to the gill-plume or ctenidium, being always placed near the base of that organ; further, Spengel has shown that the nerve-supply of this olfactory organ is always derived from the visceral loop. Accordingly, the nerve-supply affords a means of testing the conclusion that we have in Lankester’s capito-pedal bodies the rudimentary ctenidia. The accompanying diagrams (figs. 9, 10) of the nervous systems of Patella and of Haliotis, as determined by Spengel, show the identity in the origin of the nerves passing from the visceral loop to Spengel’s olfactory ganglion of the Limpet, and that of the nerves which pass from the visceral loop of Haliotis to the olfactory patch or osphradium, which lies in immediate relation on the right and on the left side to the right and left gill-plumes (ctenidia) respectively. The same diagrams serve to demonstrate the streptoneurous condition of the visceral loop in Aspidobranchia.
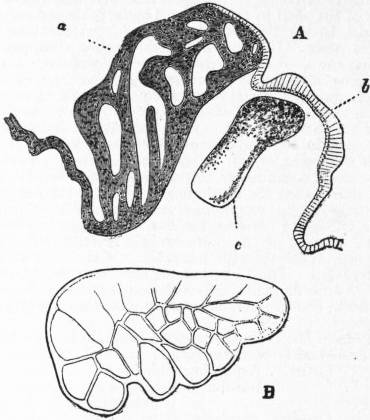 |
| Fig. 8.—A, Section in a plane vertical to the surface of the neck of Patella through a, the rudimentary ctenidium (Lankester’s organ), and b, the olfactory epithelium (osphradium); c, the olfactory (osphradial) ganglion. (After Spengel.) |
B, Surface view of a rudimentary ctenidium of Patella excised and viewed as a transparent object. (Lankester.) |
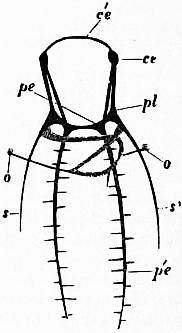 |
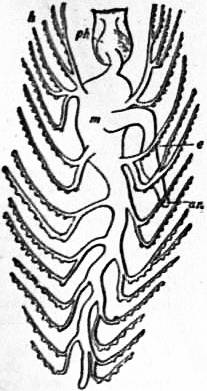
| Fig. 9.—Nervous system of Patella; the visceral loop is lightly shaded; the buccal ganglia are omitted. (After Spengel.) |
ce, Cerebral ganglia. c’e, Cerebral commissure. pl, Pleural ganglion. pe, Pedal ganglion. p′e, Pedal nerve. s, s′, Nerves (right and left) to the mantle. o, Olfactory ganglion, connected by nerve to the streptoneurous visceral loop. |
Thus, then, we find that the limpet possesses a symmetrically disposed pair of ctenidia in a rudimentary condition, and justifies its position among Aspidobranchia. At the same time it possesses a totally distinct series of functional gills, which are not derived from the modification of the typical molluscan ctenidium. These gills are in the form of delicate lamellae (fig. 4, f), which form a series extending completely round the inner face of the depending mantle-skirt. This circlet of gill-lamellae led Cuvier to class the limpets as Cyclobranchiata, and, by erroneous identification of them with the series of metamerically repeated ctenidia of Chiton, to associate the latter mollusc with the former. The gill-lamellae of Patella are processes of the mantle comparable with the plait-like folds often observed on the roof of the branchial chamber in other Gastropoda (e.g. Buccinum and Haliotis). They are termed pallial gills. The only other molluscs in which they are exactly represented are the curious Opisthobranchs Phyllidia and Pleurophyllidia (fig. 55). In these, as in Patella, the typical ctenidia are aborted, and the branchial function is assumed by close-set lamelliform processes arranged in a series beneath the mantle-skirt on either side of the foot. In fig. 4, d, the large branchial vein of Patella bringing blood from the gill-series to the heart is seen; where it crosses the series of lamellae there is a short interval devoid of lamellae.
The heart in Patella consists of a single auricle (not two as in Haliotis and Fissurella) and a ventricle; the former receives the blood from the branchial vein, the latter distributes it through a large aorta which soon leads into irregular blood-lacunae.
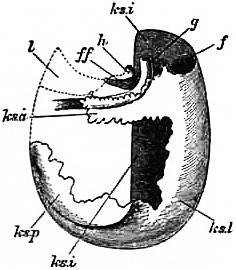
The existence of two renal organs in Patella, and their relation to the pericardium (a portion of the coelom), is important. Each renal organ is a sac lined with glandular epithelium (ciliated cell, with concretions) communicating with the exterior by its papilla, and by a narrow passage with the pericardium. The connexion with the pericardium of the smaller of the two renal organs was demonstrated by Lankester in 1867, at a time when the fact that the renal organ of the Mollusca, as a rule, opens into the pericardium, and is therefore a typical nephridium, was not known. Subsequent investigations carried on under the direction of the same naturalist have shown that the larger as well as the smaller renal sac is in communication with the pericardium. The walls of the renal sacs are deeply plaited and thrown into ridges. Below the surface these walls are excavated with blood-vessels, so that the sac is practically a series of blood-vessels covered with renal epithelium, and forming a meshwork within a space communicating with the exterior. The larger renal sac (remarkably enough, that which is aborted in other Anisopleura) extends between the liver and the integument of the visceral dome very widely. It also bends round the liver as shown in fig. 12, and forms a large sac on half of the upper surface of the muscular mass of the foot. Here it lies close upon the genital body (ovary or testis), and in such intimate relationship with it that, when ripe, the gonad bursts into the renal sac, and its products are carried to the exterior by the papilla on the right side of the anus (Robin, Dall). This fact led Cuvier erroneously to the belief that a duct existed leading from the gonad to this papilla. The position of the gonad, best seen in the diagrammatic section (fig. 13), is, as in other Aspidobranchia, devoid of a special duct communicating with the exterior. This condition, probably an archaic one, distinguishes the Aspidobranchia from other Gastropoda.
 |
|
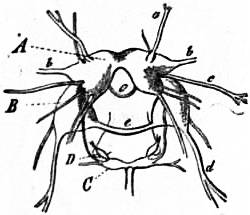
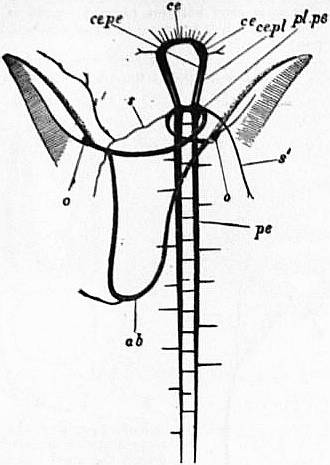
| Fig. 10.—Nervous system of Haliotis; the visceral loop is lightly shaded; the buccal ganglia are omitted. (After Spengel.) | |
ce, Cerebral ganglion. pl.pe, The fused pleural and pedal ganglia. pe, The right pedal nerve. ce.pl, The cerebro-pleural connective. |
ce.pe, The cerebro-pedal connective. s, s′, Right and left mantle nerves. ab, Abdominal ganglion or site of same. o, o, Right and left olfactory ganglia and osphardia receiving nerve from visceral loop. |
|
|
 |
|
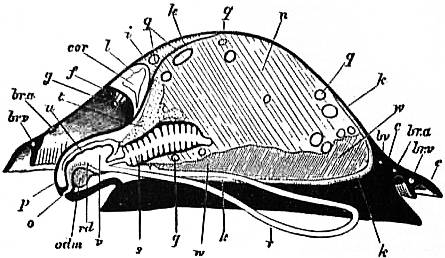
| Fig. 13.—Diagram of a vertical antero-postero median section of a Limpet. Letters as in figs. 6, 7, with following additions. (Lankester.) | |
q, Intestine in transverse section. r, Lingual sac (radular sac). rd, Radula. s, Lamellated stomach. t, Salivary gland. u, Duct of same. v, Buccal cavity |
w, Gonad. br.a, Branchial advehent vessel (artery). br.v, Branchial efferent vessel (vein). bv, Blood-vessel. odm, Muscles and cartilage of the odontophore. cor, Heart within the pericardium. |
 |
|
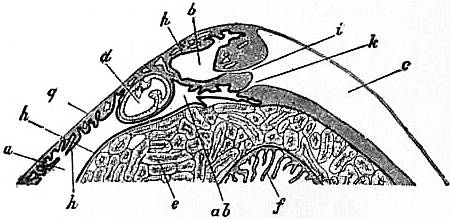
| Fig. 14.—Vertical section in a plane running right and left through the anterior part of the visceral hump of Patella to show the two renal organs and their openings into the pericardium. (J.T. Cunningham.) | |
a, Large or external or right renal organ. ab, Narrow process of the same running below the intestine and leading by k into the pericardium. b, Small or median renal organ. c, Pericardium. d, Rectum. e, Liver. |
f, Manyplies. g, Epithelium of the dorsal surface. h, Renal epithelium lining the renal sacs. i, Aperture connecting the small sac with the pericardium. k, Aperture connecting the large sac with the pericardium. |
The digestive tract of Patella offers some interesting features. The odontophore is powerfully developed; the radular sac is extraordinarily long, lying coiled in a space between the mass of the liver and the muscular foot. The radula has 160 rows of teeth with twelve teeth in each row. Two pairs of salivary ducts, each leading from a salivary gland, open into the buccal chamber. The oesophagus leads into a remarkable stomach, plaited like the manyplies of a sheep, and after this the intestine takes a very large number of turns embedded in the yellow liver, until at last it passes between the two renal sacs to the anal papilla. A curious ridge (spiral? valve) which secretes a slimy cord is found upon the inner wall of the intestine. The general structure of the Molluscan intestine has not been sufficiently investigated to render any comparison of this structure of Patella with that of other Mollusca possible. The eyes of the limpet deserve mention as examples of the most primitive kind of eye in the Molluscan series. They are found one on each cephalic tentacle, and are simply minute open pits or depressions of the epidermis, the epidermic cells lining them being pigmented and connected with nerves (compare fig. 14, art. Cephalopoda). The limpet breeds upon the southern English coast in the early part of April, but its development has not been followed. It has simply been traced as far as the formation of a diblastula which acquires a ciliated band, and becomes a nearly spherical trochosphere. It is probable that the limpet takes several years to attain full growth, and during that period it frequents the same spot, which becomes gradually sunk below the surrounding surface, especially if the rock be carbonate of lime. At low tide the limpet (being a strictly intertidal organism) is exposed to the air, and (according to trustworthy observers) quits its attachment and walks away in search of food (minute encrusting algae), and then once more returns to the identical spot, not an inch in diameter, which belongs, as it were, to it. Several million limpets—twelve million in Berwickshire alone—are annually used on the east coast of Britain as bait.
Sub-order 1. Docoglossa.—Nervous system without dialyneury. Eyes are open invaginations without crystalline lens. Two osphradia present but no hypobranchial glands nor operculum. Teeth of radula beam-like, and at most three marginal teeth on each side. Heart has only a single auricle, neither heart nor pericardium traversed by rectum. Shell conical without spire.
Fam. 1.—Acmaeidae. A single bipectinate ctenidium on left side. Acmaea, without pallial branchiae, British. Scurria, with pallial branchiae in a circle beneath the mantle.
Fam. 2.—Tryblidiidae. Muscle scar divided into numerous impressions. Tryblidium, Silurian.
Fam. 3.—Patellidae. No ctenidia but pallial branchiae in a circle between mantle and foot. Patella, pallial branchiae forming a complete circle, no epipodial tentacles, British. Ancistromesus, radula with median central tooth. Nacella, epipodial tentacles present. Helcion, circlet of branchiae interrupted anteriorly, British.
Fam. 4.—Lepetidae. Neither ctenidia nor pallial branchiae. Lepeta, without eyes. Pilidium. Propilidium.
Fam. 5.—Bathysciadidae. Hermaphrodite; head with appendage on right side; radula without central tooth. Bathysciadium, abyssal.
Sub-order 2. Rhipidoglossa.—Aspidobranchia with a palliovisceral anastomosis (dialyneurous); eye-vesicle closed, with crystalline lens; ctenidia, osphradia and hypobranchial glands paired or single. Radula with very numerous marginal teeth arranged like the rays of a fan. Heart with two auricles; ventricle traversed by the rectum, except in the Helicinidae. An epipodial ridge on each side of the foot and cephalic expansions between the tentacles often present.
Fam. 1.—Pleurotomariidae. Shell spiral; mantle and shell with an anterior fissure; two ctenidia; a horny operculum. Pleurotomaria, epipodium without tentacles. Genus includes several hundred extinct species ranging from the Silurian to the Tertiary. Five living species from the Antilles, Japan and the Moluccas. Moluccan species is 19 cm. in height.
Fam. 2.—Bellerophontidae. 300 species, all fossil, from Cambrian to Trias.
Fam. 3.—Euomphalidae. Also extinct, from Cambrian to Cretaceous.
Fam. 4.—Haliotidae. Spire of shell much reduced; two bipectinate ctenidia, the right being the smaller; no operculum. Haliotis.
Fam. 5.—Velainiellidae, an extinct family from the Eocene.
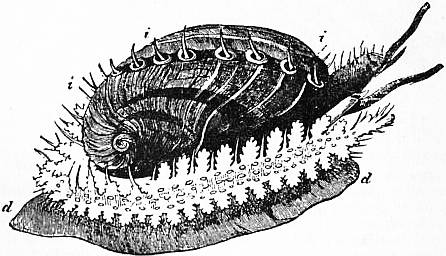 |
| Fig. 15.—Halio tistuberculata. d, Foot; i, tentacular processes of the mantle. (From Owen, after Cuvier.) |
Fam. 6.—Fissurellidae. Shell conical; slit or hole in anterior part of mantle; two symmetrical ctenidia; no operculum. Emarginula, mantle and shell with a slit, British. Scutum, mantle split anteriorly and reflected over shell, which has no slit. Puncturella, mantle and shell with a foramen in front of the apex, British. Fissurella, mantle and shell perforated at apex, British.
Fam. 7.—Cocculinidae. Shell conical, symmetrical, without slit or perforation. Cocculina, abyssal.
Fam. 8.—Trochidae. Shell spirally coiled; a single ctenidium; eyes perforated; a horny operculum; lobes between the tentacles. Trochus, shell umbilicated, spire pointed and prominent, British. Monodonta, no jaws, spire not prominent, no umbilicus, columella toothed. Gibbula, with jaws, three pairs of epipodial cirri without pigment spots at their bases, British. Margarita, five to seven pairs of epipodial cirri with a pigment spot at base of each.
|
|
Fam. 9.—Stomatellidae. Spire of shell much reduced; a single ctenidium. Stomatella, foot truncated posteriorly, an operculum present, no epipodial tentacles. Gena, foot elongated posteriorly, no operculum.
Fam. 10.—Delphinulidae. Shell spirally coiled; operculum horny; intertentacular lobes absent. Delphinula.
Fam. 11.—Liotiidae, shell globular, margin of aperture thickened. Liotia.
Fam. 12.—Cyclostrematidae. Shell flattened, umbilicated; foot anteriorly truncated with angles produced into lobes. Cyclostrema. Teinostoma.
Fam. 13.—Trochonematidae. All extinct, Cambrian to Cretaceous.
Fam. 14.—Turbinidae. Shell spirally coiled; epipodial tentacles present; operculum thick and calcareous. Turbo. Astralium. Molleria. Cyclonema.
Fam. 15.—Phasianellidae. Shell not nacreous, without umbilicus, with prominent spire and polished surface. Phasianella.
Fam. 16.—Umboniidae. Shell flattened, not umbilicated, generally smooth; operculum horny. Umbonium. Isanda.
Fam. 17.—Neritopsidae. Shell semi-globular, with short spire; operculum calcareous, not spiral. Neritopsis. Naticopsis, extinct.
Fam. 18.—Macluritidae. Extinct, Cambrian and Silurian.
Fam. 19.—Neritidae. Shell with very low spire, without umbilicus, internal partitions frequently absorbed; a single ctenidium; a cephalic penis present. Nerita, marine. Neritina, freshwater, British. Septaria, shell boat-shaped.
Fam. 20.—Titiscaniidae. Without shell and operculum, but with pallial cavity and ctenidium. Titiscania, Pacific.
Fam. 21.—Helicinidae. No ctenidium, but a pulmonary cavity; heart with a single auricle, not traversed by the rectum. Helicina. Eutrochatella. Stoastoma. Bourceria.
Fam. 22.—Hydrocenidae. No ctenidium, but a pulmonary cavity; operculum with an apophysis. Hydrocena, Dalmatia.
Fam. 23.—Proserpinidae. No operculum. Proserpina, Central America.
Order 2. Pectinibranchia.—In this order there is no longer any trace of bilateral symmetry in the circulatory, respiratory and excretory organs, the topographically right half of the pallial complex having completely disappeared, except the right kidney, which is represented by the genital duct. There is usually a penis in the male. The ctenidium is monopectinate and attached to the mantle along its whole length, except in Adeorbis and Valvata; in the latter alone it is bipectinate. There is a single well-developed, often pectinated osphradium. The eye is always a closed vesicle, and the internal cornea is extensive. In the radula there is a single central tooth or none.
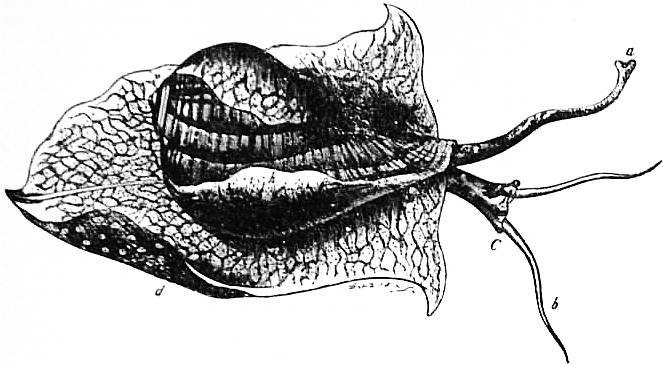 |
|
| Fig. 18.—Animal and shell of Pyrula laevigata. (From Owen.) | |
a, Siphon. b, Head-tentacles. C, Head, the letter placed near the right eye. |
d, The foot, expanded as in crawling. h, The mantle-skirt reflected over the sides of the shell. |
The former classification into Holochlamyda, Pneumochlamyda and Siphonochlamyda has been abandoned, as it was founded on adaptive characters not always indicative of true affinities. The order is now divided into two sub-orders: the Taenioglossa, in which there are three teeth on each side of the median tooth of the radula, and the Stenoglossa, in which there is only one tooth on each side of the median tooth. In the latter a pallial siphon, a well-developed proboscis and an unpaired oesophageal gland are always present, in the former they are usually absent. The siphon is an incompletely tubular outgrowth of the mantle margin on the left side, contained in a corresponding outgrowth of the edge of the shell-mouth, and serving to conduct water to the respiratory cavity.
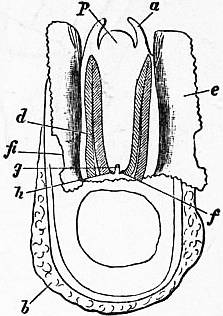
The condition usually spoken of as a “proboscis” appears to be derived from the condition of a simple rostrum (having the mouth at its extremity) by the process of incomplete introversion of that simple rostrum. There is no reason in the actual significance of the word why the term “proboscis” should be applied to an alternately introversible and eversible tube connected with an animal’s body, and yet such is a very customary use of the term. The introversible tube may be completely closed, as in the “proboscis” of Nemertine worms, or it may have a passage in it leading into a non-eversible oesophagus, as in the present case, and in the case of the eversible pharynx of the predatory Chaetopod worms. The diagrams here introduced (fig. 19) are intended to show certain important distinctions which obtain amongst the various “introverts,” or intro- and e-versible tubes so frequently met with in animal bodies. Supposing the tube to be completely introverted and to commence its eversion, we then find that eversion may take place, either by a forward movement of the side of the tube near its attached base, as in the proboscis of the Nemertine worms, the pharynx of Chaetopods and the eye-tentacle of Gastropods, or by a forward movement of the inverted apex of the tube, as in the proboscis of the Rhabdocoel Planarians, and in that of Gastropods here under consideration. The former case we call “pleurecbolic” (fig. 19, A, B, C, H, I, K), the latter “acrecbolic” tubes or introverts (fig. 19, D, E, F, G). It is clear that, if we start from the condition of full eversion of the tube and watch the process of introversion, we shall find that the pleurecbolic variety is introverted by the apex of the tube sinking inwards; it may be called acrembolic, whilst conversely the acrecbolic tubes are pleurembolic. Further, it is obvious enough that the process either of introversion or of eversion of the tube may be arrested at any point, by the development of fibres connecting the wall of the introverted tube with the wall of the body, or with an axial structure such as the oesophagus; on the other hand, the range of movement of the tubular introvert may be unlimited or complete. The acrembolic proboscis or frontal introvert of the Nemertine worms has a complete range. So has the acrembolic pharynx of Chaetopods, if we consider the organ as terminating at that point where the jaws are placed and the oesophagus commences. So too the acrembolic eye-tentacle of the snail has a complete range of movement, and also the pleurembolic proboscis of the Rhabdocoel prostoma. The introverted rostrum of the Pectinibranch Gastropods presents in contrast to these a limited range of movement. The “introvert” in these Gastropods is not the pharynx as in the Chaetopod worms, but a prae-oral structure, its apical limit being formed by the true lips and jaws, whilst the apical limit of the Chaetopod’s introvert is formed by the jaws placed at the junction of pharynx and oesophagus, so that the Chaetopod’s introvert is part of the stomodaeum or fore-gut, whilst that of the Gastropod is external to the alimentary canal altogether, being in front of the mouth, not behind it, as is the Chaetopod’s. Further, the Gastropod’s introvert is pleurembolic (and therefore acrecbolic), and is limited both in eversion and in introversion; it cannot be completely everted owing to the muscular bands (fig. 19, G), nor can it be fully introverted owing to the bands (fig. 19, F) which tie the axial pharynx to the adjacent wall of the apical part of the introvert. As in all such intro- and e-versible organs, eversion of the Gastropod proboscis is effected by pressure communicated by the muscular body-wall to the liquid contents (blood) of the body-space, accompanied by the relaxation of the muscles which directly pull upon either the sides or the apex of the tubular organ. The inversion of the proboscis is effected directly by the contraction of these muscles. In various members of the Pectinibranchia the mouth-bearing cylinder is introversible (i.e. is a proboscis)—with rare exceptions these forms have a siphonate mantle-skirt. On the other hand, many which have a siphonate mantle-skirt are not provided with an introversible mouth-bearing cylinder, but have a simple non-introversible rostrum, as it has been termed, which is also the condition presented by the mouth-bearing region in nearly all other Gastropoda. One of the best examples of the introversible mouth-cylinder or proboscis which can be found is that of the common whelk (Buccinum undatum) and its immediate allies. In fig. 23 the proboscis is seen in an everted state; it is only so carried when feeding, being withdrawn when the animal is at rest. Probably its use is to enable the animal to introduce its rasping and licking apparatus into very narrow apertures for the purposes of feeding, e.g. into a small hole bored in the shell of another mollusc.
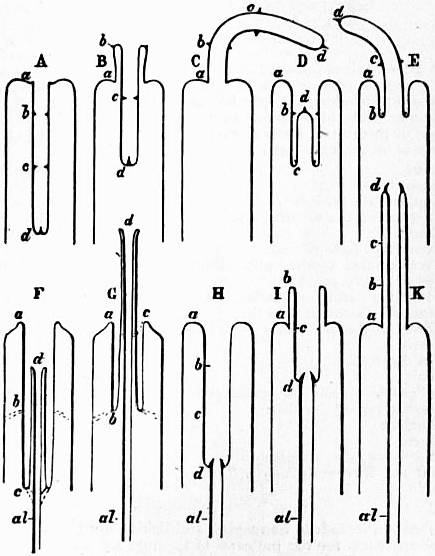 |
| Fig. 19.—Diagrams explanatory of the nature of so-called proboscides or “introverts.” (Lankester.) |
A, Simple introvert completely introverted.
B, The same, partially everted by eversion of the sides, as in the Nemertine proboscis and Gastropod eye-tentacle = pleurecbolic.
C, The same, fully everted.
D, E, A similar simple introvert in course of eversion by the forward movement, not of its sides, but of its apex, as in the proboscidean Rhabdocoels = acrecbolic.
F, Acrecbolic (= pleurembolic) introvert, formed by the snout of the proboscidiferous Gastropod. al, alimentary canal; d, the true mouth. The introvert is not a simple one with complete range both in eversion and introversion, but is arrested in introversion by the fibrous bands at c, and similarly in eversion by the fibrous bands at b.
G, The acrecbolic snout of a proboscidiferous Gastropod, arrested short of complete eversion by the fibrous band b.
H, The acrembolic (= pleurecbolic) pharynx of a Chaetopod fully introverted. al, alimentary canal; at d, the jaws; at a, the mouth; therefore a to d is stomodaeum, whereas in the Gastropod (F) a to d is inverted body-surface.
I, Partial eversion of H.
K, Complete eversion of H.
|
|
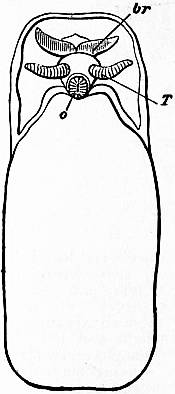
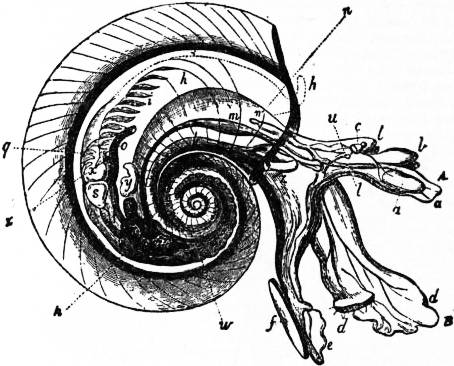
The very large assemblage of forms coming under this order comprises the most highly developed predaceous sea-snails, numerous vegetarian species, a considerable number of freshwater and some terrestrial forms. The partial dissection of a male specimen of the common periwinkle, Littorina littoralis, drawn in fig. 20, will serve to exhibit the disposition of viscera which prevails in the group. The branchial chamber formed by the mantle-skirt overhanging the head has been exposed by cutting along a line extending backward from the letters vd to the base of the columella muscle mc, and the whole roof of the chamber thus detached from the right side of the animal’s neck has been thrown over to the left, showing the organs which lie upon the roof. No opening into the body-cavity has been made; the organs which lie in the coiled visceral hump show through its transparent walls. The head is seen in front resting on the foot and carrying a median non-retractile snout or rostrum, and a pair of cephalic tentacles at the base of each of which is an eye. In many Gastropoda the eyes are not thus sessile but raised upon special eye-tentacles (figs. 25, 56). To the right of the head is seen the muscular penis p, close to the termination of the vas deferens (spermatic duct) vd. The testis t occupies a median position in the coiled visceral mass. Behind the penis on the same side is the hook-like columella muscle, a development of the retractor muscle of the foot, which clings to the spiral column or columella of the shell (see fig. 33). This columella muscle is the same thing as the muscles adhering to the shell in Patella, and the posterior adductor of Lamellibranchs.
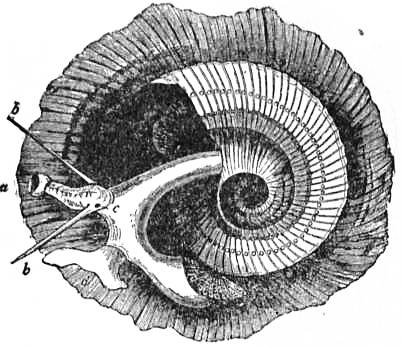
The surface of the neck is covered by integument forming the floor of the branchial cavity. It has not been cut into. Of the organs lying on the reflected mantle-skirt, that which in the natural state lay nearest to the vas deferens on the right side of the median line of the roof of the branchial chamber is the rectum i′, ending in the anus a. It can be traced back to the intestine i near the surface of the visceral hump, and it is found that the apex of the coil formed by the hump is occupied by the liver h and the stomach v. Pharynx and oesophagus are concealed in the head. The enlarged glandular structure of the walls of the rectum is frequent in the Pectinibranchia, as is also though not universal the gland marked y, next to the rectum. It is the adrectal gland, and in the genera Murex and Purpura secretes a colourless liquid which turns purple upon exposure to the atmosphere, and was used by the ancients as a dye. Near this and less advanced into the branchial chamber is the single renal organ or nephridium r with its opening to the exterior r′. Internally this glandular sac presents a second slit or aperture which leads into the pericardium (as is now found to be the case in all Mollusca). The heart c lying in the pericardium is seen in close proximity to the renal organ, and consists of a single auricle receiving blood from the gill, and of a single ventricle which pumps it through the body by an anterior and posterior aorta. The surface x of the mantle between the rectum and the gill-plume is thrown into folds which in many sea-snails (whelks or Buccinidae, &c.) are very strongly developed. The whole of this surface appears to be active in the secretion of a mucous-like substance. The single gill-plume br lies to the left of the median line in natural position. It corresponds to the right of the two primitive ctenidia in the untwisted archaic condition of the molluscan body, and does not project freely into the branchial cavity, but its axis is attached (by concrescence) to the mantle-skirt (roof of the branchial chamber). It is rare for the gill-plume of a Pectinibranch Gastropod to stand out freely as a plume, but occasionally this more archaic condition is exhibited as in Valvata (fig. 30). Next beyond (to the left of) the gill-plume we find the so-called parabranchia, which is here simple, but sometimes lamellated as in Purpura (fig. 22). This organ has, without reason, been supposed to represent the second ctenidium of the typical mollusc, which it cannot do on account of its position. It should be to the right of the anus were this the case. Spengel showed that the parabranchia of Gastropods is the typical olfactory organ or osphradium in a highly developed condition. The minute structure of the epithelium which clothes it, as well as the origin of the nerve which is distributed to the parabranchia, proves it to be the same organ which is found universally in molluscs at the base of each gill-plume, and tests the indrawn current of water by the sense of smell. The nerve to this organ is given off from the superior (original right, see fig. 3) visceral ganglion.
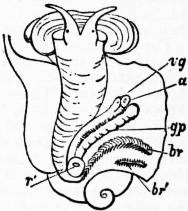 |
| Fig. 22.—Female of Purpura lapillus removed from its shell; the mantle-skirt cut along its left line of attachment and thrown over to the right side of the animal so as to expose the organs on its inner face. |
a, Anus. vg, Vagina. gp, Adrectal purpuriparous gland. r′, Aperture of the nephridium (kidney). br, Ctenidium (branchial plume). br′, Parabranchia (= the comb-like osphradium or olfactory organ). |
The figures which are given here of various Pectinibranchia are in most cases sufficiently explained by the references attached to them. As an excellent general type of the nervous system, attention may be directed to that of Paludina drawn in fig. 21. On the whole the ganglia are strongly individualized in the Pectinibranchia, nerve-cell tissue being concentrated in the ganglia and absent from the cords. At the same time, the junction of the visceral loop above the intestine prevents in all Streptoneura the shortening of the visceral loop, and it is rare to find a fusion of the visceral ganglia with either pleural, pedal or cerebral—a fusion which can and does take place where the visceral loop is not above but below the intestine, e.g. in the Euthyneura (fig. 48), Cephalopoda and Lamellibranchia. As contrasted with the Aspidobranchia, we find that in the Pectinibranchia the pedal nerves are distinctly nerves given off from the pedal ganglia, rather than cord-like nerve-tracts containing both nerve-cells or ganglionic elements and nerve-fibres. Yet in some Pectinibranchia (Paludina) a ladder-like arrangement of the two pedal nerves and their lateral branches has been detected. The histology of the nervous system of Mollusca has yet to be seriously inquired into.
The alimentary canal of the Pectinibranchia presents little diversity of character, except in so far as the buccal region is concerned. Salivary glands are present, and in some carnivorous forms (Dolium) these secrete free sulphuric acid (as much as 2% is present in the secretion), which assists the animal in boring holes by means of its rasping tongue through the shells of other molluscs upon which it preys. A crop-like dilatation of the gut and a recurved intestine, embedded in the compact yellowish-brown liver, the ducts of which open into it, form the rest of the digestive tract and occupy a large bulk of the visceral hump. The buccal region presents a pair of shelly jaws placed laterally upon the lips, and a wide range of variation in the form of the denticles of the lingual ribbon or radula.
Well-developed glandular invaginations occur in different positions on the foot in Pectinibranchia. The most important of these opens by the ventral pedal pore, situated in the median line in the anterior half of the foot. This organ is probably homologous with the byssogenous gland of Lamellibranchs. The aperture, which was formerly supposed to be an aquiferous pore, leads into an extensive and often ramified cavity surrounded by glandular tubules. The gland has been found in both sub-orders of the Pectinibranchia, in Cyclostoma and Cypraea among the Taenioglossa, in Hemifusus, Cassis, Nassa, Murex, Fasciolariidae, Turbinellidae, Olividae, Marginellidae and Conidae among the Stenoglossa. It was discovered by J.T. Cunningham that in Buccinum the egg-capsules are formed by this pedal gland and not by any accessory organ of the generative system. Such horny egg-capsules doubtless have the same origin in all other species in which they occur, e.g. Fusus, Pyrula, Purpura, Murex, Nassa, Trophon, Voluta, &c. The float of the pelagic Janthina, to which the egg-capsules are attached, probably is also formed by the secretion of the pedal gland.
 |
|
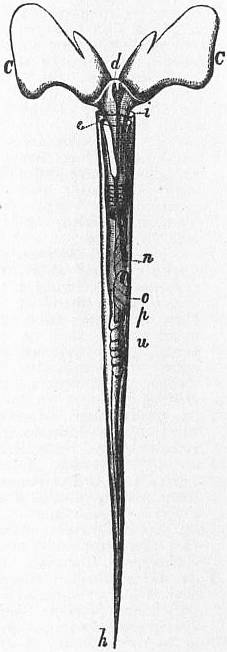
| Fig. 23.—A, Triton variegatum, to show the proboscis or buccal introvert (e) in a state of eversion. | |
a, Siphonal notch of the shell occupied by the siphonal fold of the mantle-skirt (Siphonochlamyda). b, Edge of the mantle-skirt resting on the shell. c, Cephalic eye. d, Cephalic tentacle. e, Everted buccal introvert (proboscis). |
f, Foot. g, Operculum. h, Penis. i, Under surface of the mantle-skirt forming the roof of the sub-pallial chamber. |
| B, Sole of the foot of Pyrula tuba, to show a, the pore usually said to be “aquiferous” but probably the orifice of a gland; b, median line of foot. | |
Other glands opening on or near the foot are: (1) The suprapedal gland opening in the middle line between the snout and the anterior border of the foot. It is most commonly found in sessile forms and in terrestrial genera such as Cyclostoma; (2) the anterior pedal gland opening into the anterior groove of the foot, generally present in aquatic species; (3) dorsal posterior mucous glands in certain Cyclostomatidae.
The foot of the Pectinibranchia, unlike the simple muscular disk of the Isopleura and Aspidobranchia, is very often divided into lobes, a fore, middle and hind lobe (pro-, meso- and meta-podium, see figs. 24 and 25). Very usually, but not universally, the metapodium carries an operculum. The division of the foot into lobes is a simple case of that much greater elaboration or breaking up into processes and regions which it undergoes in the class Cephalopoda. Even among some Gastropoda (viz. the Opisthobranchia) we find the lobation of the foot still further carried out by the development of lateral lobes, the parapodia, whilst there are many Pectinibranchia, on the other hand, in which the foot has a simple oblong form without any trace of lobes.
The development of the Pectinibranchia has been followed in several examples, e.g. Paludina, Purpura, Nassa, Vermetus, Neritina. As in other Molluscan groups, we find a wide variation in the early process of the formation of the first embryonic cells, and their arrangement as a diblastula, dependent on the greater or less amount of food-yolk which is present in the egg-cell when it commences its embryonic changes. In fig. 26 the early stages of Paludina vivipara are represented. There is but very little food-material in the egg of this Pectinibranch, and consequently the diblastula forms by invagination; the blastopore or orifice of invagination coincides with the anus, and never closes entirely. A well-marked trochosphere is formed by the development of an equatorial ciliated band; and subsequently, by the disproportionate growth of the lower hemisphere, the trochosphere becomes a veliger. The primitive shell-sac or shell-gland is well marked at this stage, and the pharynx is seen as a new ingrowth (the stomodaeum), about to fuse with and open into the primitively invaginated arch-enteron (fig. 26, F).
 |
|
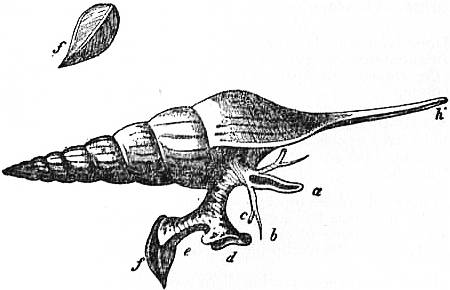
| Fig. 24.—Animal and shell of Phorus exutus. | |
a, Snout (not introversible). b, Cephalic tentacles. c, Right eye. |
d, Pro- and meso-podium; to the right of this is seen the metapodium bearing the sculptured operculum. |
 |
|
| Fig. 25.—Animal and shell of Rostellaria rectirostris. (From Owen.) | |
a, Snout or rostrum. b, Cephalic tentacle. c, Eye. d, Propodium and mesopodium. |
e, Metapodium. f, Operculum. h′, Prolonged siphonal notch of the shell occupied by the siphon, or trough-like process of the mantle-skirt. |
In other Pectinibranchia (and such variations are representative for all Mollusca, and not characteristic only of Pectinibranchia) we find that there is a very unequal division of the egg-cell at the commencement of embryonic development, as in Nassa. Consequently there is, strictly speaking, no invagination (emboly), but an overgrowth (epiboly) of the smaller cells to enclose the larger. The general features of this process and of the relation of the blastopore to mouth and anus have been explained in treating of the development of Mollusca generally. In such cases the blastopore may entirely close, and both mouth and anus develop as new ingrowths (stomodaeum and proctodaeum), whilst, according to the observations of N. Bobretzky, the closed blastopore may coincide in position with the mouth in some instances (Nassa, &c.), instead of with the anus. But in these epibolic forms, just as in the embolic Paludina, the embryo proceeds to develop its ciliated band and shell-gland, passing through the earlier condition of a trochosphere to that of the veliger. In the veliger stage many Pectinibranchia (Purpura, Nassa, &c.) exhibit, in the dorsal region behind the head, a contractile area of the body-wall. This acts as a larval heart, but ceases to pulsate after a time. Similar rhythmically contractile areas are found on the foot of the embryo Pulmonate Limax and on the yolk-sac (distended foot-surface) of the Cephalopod Loligo. The preconchylian invagination or shell-gland is formed in the embryo behind the velum, on the surface opposite the blastopore. It is surrounded by a ridge of cells which gradually extends over the visceral sac and secretes the shell. In forms which are naked in the adult state, the shell falls off soon after the reduction of the velum, but in Cenia, Runcina and Vaginula the shell-gland and shell are not developed, and the young animal when hatched has already the naked form of the adult.
 |
|
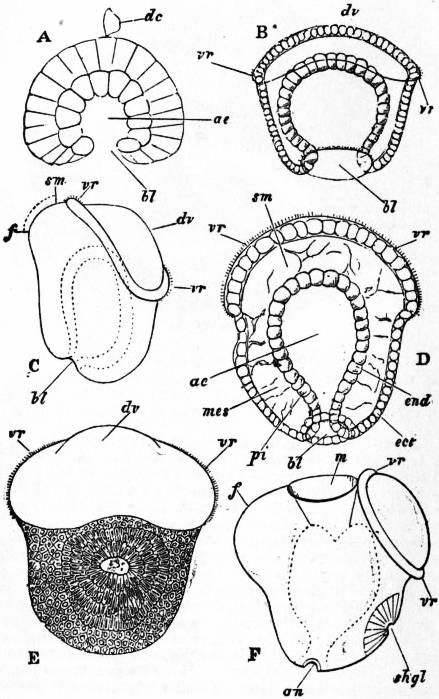
| Fig. 26.—Development of the River-Snail, Paludina vivipara. (After Lankester, 17.) | |
dc, Directive corpuscle (outcast cell). ae, Arch-enteron or cavity lined by the enteric cell-layer or endoderm. bl, Blastopore. vr, Velum or circlet of ciliated cells. dv, Velar area or cephalic dome. sm, Site of the as yet unformed mouth. |
f, Foot. mes, Rudiments of the skeleto-trophic tissues. pi, The pedicle of invagination, the future rectum. shgl, The primitive shell-sac or shell-gland. m, Mouth. an, Anus. |
A, Diblastula phase (optical section).
B, The diblastula has become a trochosphere by the development of the ciliated ring vr (optical section).
C, Side view of the trochosphere with commencing formation of the foot.
D, Further advanced trochosphere (optical section).
E, The trochosphere passing to the veliger stage, dorsal view showing the formation of the primitive shell-sac.
F, Side view of the same, showing foot, shell-sac (shgl), velum (vr), mouth and anus.
N.B.—In this development the blastopore is not elongated; it persists as the anus. The mouth and stomodaeum form independently of the blastopore.
One further feature of the development of the Pectinibranchia deserves special mention. Many Gastropoda deposit their eggs, after fertilization, enclosed in capsules; others, as Paludina, are viviparous; others, again, as the Zygobranchia, agree with the Lamellibranch Conchifera (the bivalves) in having simple exits for the ova without glandular walls, and therefore discharge their eggs unenclosed in capsules freely into the sea-water; such unencapsuled eggs are merely enclosed each in its own delicate chorion. When egg-capsules are formed they are often of large size, have tough walls, and in each capsule are several eggs floating in a viscid fluid. In some cases all the eggs in a capsule develop; in other cases one egg only in a capsule (Neritina), or a small proportion (Purpura, Buccinum), advance in development; the rest are arrested either after the first process of cell-division (cleavage) or before that process. The arrested embryos or eggs are then swallowed and digested by those in the same capsule which have advanced in development. This is clearly the same process in essence as that of the formation of a vitellogenous gland from part of the primitive ovary, or of the feeding of an ovarian egg by the absorption of neighbouring potential eggs; but here the period at which the sacrifice of one egg to another takes place is somewhat late. What it is that determines the arrest of some eggs and the progressive development of others in the same capsule is at present unknown.
 |
|
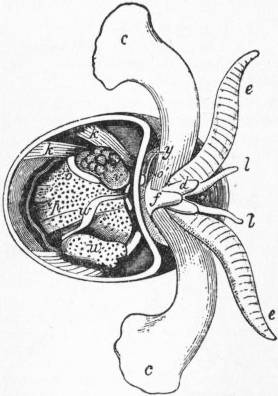
| Fig. 27.—Oxygyrus Keraudrenii. (From Owen.) | |
a, Mouth and odontophore. b, Cephalic tentacles. c, Eye. d, Propodium (B) and mesopodium. e, Metapodium. f, Operculum. h, Mantle-chamber. i, Ctenidium (gill-plume). k, Retractor muscle of foot. l, Optic tentacle. m, Stomach. |
n, Dorsal surface overhung by the mantle-skirt; the letter is close to the salivary gland. o, Rectum and anus. p, Liver. q, Renal organ (nephridium). s, Ventricle. u, The otocyst attached to the cerebral ganglion. w, Testis. x, Auricle of the heart. y, Vesicle on genital duct. z, Penis. |
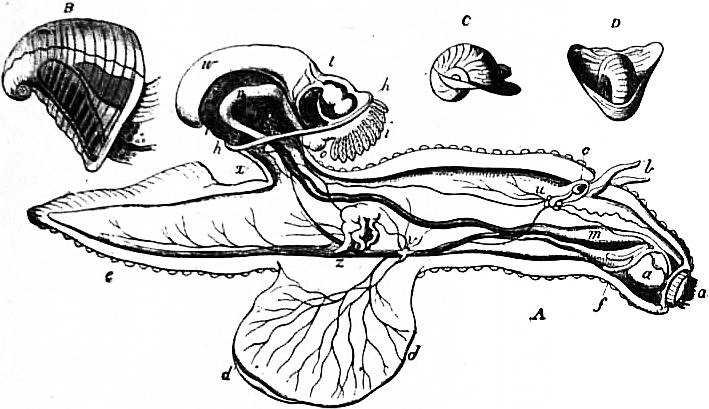
In the tribe of Pectinibranchia called Heteropoda the foot takes the form of a swimming organ. The nervous system and sense organs are highly developed. The odontophore also is remarkably developed, its lateral teeth being mobile, and it serves as an efficient organ for attacking the other pelagic forms on which the Heteropoda prey. The sexes are distinct, as in all Streptoneura; and genital ducts and accessory glands and pouches are present, as in all Pectinibranchia. The Heteropoda exhibit a series of modifications in the form and proportions of the visceral mass and foot, leading from a condition readily comparable with that of a typical Pectinibranch such as Rostellaria, with the three regions of the foot strongly marked and a coiled visceral hump of the usual proportions, up to a condition in which the whole body is of a tapering cylindrical shape, the foot a plate-like vertical fin, and the visceral hump almost completely atrophied. Three steps of this modification may be distinguished as three families:—Atlantidae, Carinariidae and Pterotrachaeidae. They are true Pectinibranchia which have taken to a pelagic life, and the peculiarities of structure which they exhibit are strictly adaptations consequent upon their changed mode of life. Such adaptations are the transparency and colourlessness of the tissues, and the modifications of the foot, which still shows in Atlanta the form common in Pectinibranchia (compare fig. 27 and fig. 24). The cylindrical body of Pterotrachaea is paralleled by the slug-like forms of Euthyneura. J.W. Spengel has shown that the visceral loop of the Heteropoda is streptoneurous. Special to the Heteropoda is the high elaboration of the lingual ribbon, and, as an agreement with some of the opisthobranchiate Euthyneura, but as a difference from the Pectinibranchia, we find the otocysts closely attached to the cerebral ganglia. This is, however, less of a difference than it was at one time supposed to be, for it has been shown by H. Lacaze-Duthiers, and also by F. Leydig, that the otocysts of Pectinibranchia even when lying close upon the pedal ganglion (as in fig. 21) yet receive their special nerve (which can sometimes be readily isolated) from the cerebral ganglion (see fig. 11). Accordingly the difference is one of position of the otocyst and not of its nerve-supply. The Heteropoda are further remarkable for the high development of their cephalic eyes, and for the typical character of their osphradium (Spengel’s olfactory organ). This is a groove, the edges of which are raised and ciliated, lying near the branchial plume in the genera which possess that organ, whilst in Firoloida, which has no branchial plume, the osphradium occupies a corresponding position. Beneath the ciliated groove is placed an elongated ganglion (olfactory ganglion) connected by a nerve to the supra-intestinal (therefore the primitively dextral) ganglion of the long visceral nerve-loop, the strands of which cross one another—this being characteristic of Streptoneura (Spengel).
 |
|
| Fig. 28.—Carinaria mediterranea. (From Owen.) A, The animal. B, The shell removed. C, D, Two views of the shell of Cardiopoda. |
|
a, Mouth and odontophore. b, Cephalic tentacles. c, Eye. d, The fin-like mesopodium. d’, Its sucker. e, Metapodium. f, Salivary glands. h, Border of the mantle-flap. i, Ctenidium (gill-plume). m, Stomach. |
n, Intestine. o, Anus. p, Liver. t, Aorta, springing from the ventricle. u, Cerebral ganglion. v, Pleural and pedal ganglion. w, Testis. x, Visceral ganglion. y, Vesicula seminalis. z, Penis. |
The Heteropoda belong to the “pelagic fauna” occurring near the surface in the Mediterranean and great oceans in company with the Pteropoda, the Siphonophorous Hydrozoa, Salpae, Leptocephali, and other specially-modified transparent swimming representatives of various groups of the animal kingdom. In development they pass through the typical trochosphere and veliger stages provided with boat-like shell.
Sub-order 1.—Taenioglossa. Radula with a median tooth and three teeth on each side of it. Formula 3 : 1 : 3.
Tribe 1.—Platypoda. Normal Taenioglossa of creeping habit. The foot is flattened ventrally, at all events in its anterior part (Strombidae). Otocysts situated close to the pedal nerve-centres. Accessory organs are rarely found on the genital ducts, but occur in Paludina, Cyclostoma, Naticidae, Calyptraeidae, &c. Mandibles usually present. This is the largest group of Mollusca, including nearly sixty families, some of which are insufficiently known from the anatomical point of view.
Fam. 1.—Paludinidae. Pedal centres in the form of ganglionated cords; kidney provided with a ureter; viviparous; fluviatile. Paludina. Neothauma, from Lake Tanganyika. Tylopoma, extinct, Tertiary.
 |
|
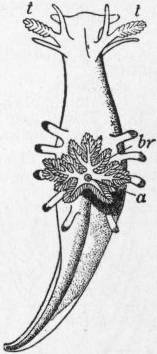
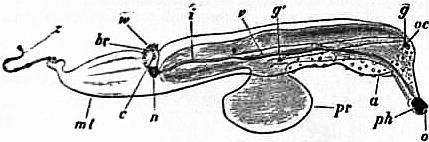
| Fig. 29.—Pterotrachea mutica seen from the right side. (After Keferstein.) | |
a, Pouch for reception of the snout when retracted. c, Pericardium. ph, Pharynx. oc, Cephalic eye. g, Cerebral ganglion. g’, Pleuro-pedal ganglion. pr, Foot (mesopodium). |
v, Stomach. i, Intestine. n, So-called nucleus. br, Branchial plume (ctenidium). w, Osphradium. mt, Foot (metapodium). z, Caudal appendage. |
Fam. 2.—Cyclophoridae. No ctenidium, pallial cavity transformed into a lung; aperture of shell circular; terrestrial. Pomatias, shell turriculated. Diplommatina. Hybocystis. Cyclophorus, shell umbilicated, with a short spire and horny operculum. Cyclosurus, shell uncoiled. Dermatocera, foot with a horn-shaped protuberance at its posterior end. Spiraculum.
Fam. 3.—Ampullariidae. To the left of the ctenidium a pulmonary sac, separated from it by an incomplete septum, amphibious. Ampullaria, shell dextral, coiled. Lanistes, shell sinistral, spire short or obsolete. Meladomus.
Fam. 4.—Littorinidae. Oesophageal pouches present; pedal nerve-centres concentrated; a pedal penis near the right tentacle. Littorina, shell not umbilicated, littoral habit. Lacuna, foot with two posterior appendages, marine, entirely aquatic. Cremnoconchus, entirely aerial, Indian. Risella. Tectarius.
Fam. 5.—Fossaridae. Head with two lobes in some Rhipidoglossa. Fossaria.
Fam. 6.—Purpurinidae, extinct.
Fam. 7.—Planaxidae. Shell with pointed spire; a short pallial siphon. Planaxis.
Fam. 8.—Cyclostomatidae. Pallial cavity transformed into a lung; pedal centres concentrated; a deep pedal groove. Cyclostoma, shell turbinated, operculum calcareous, British. Omphalotropis.
Fam. 9.—Aciculidae. Pallial cavity transformed into a lung; operculum horny; shell narrow and elongated. Acicula.
Fam. 10.—Valvatidae. Ctenidium bipectinate, free; hermaphrodite; fluviatile. Valvata, British.
Fam. 11.—Rissoidae. Epipodial filaments present; one or two pallial tentacles. Rissoa. Rissoina. Stiva.
Fam. 12.—Litiopidae. An epipodium bearing three pairs of tentacles and an operculigerous lobe with two appendages; inhabitants of the Sargasso weed. Litiopa.
Fam. 13.—Adeorbiidae. Mantle with two posterior appendages; ctenidium large and capable of protrusion from pallial cavity. Adeorbis, British.
Fam. 14.—Jeffreysiidae. Head with two long labial palps; shell ovoid; operculum horny, semicircular, carinated. Jeffreysia.
Fam. 15.—Homalogyridae. Shell flattened; no cephalic tentacles. Homalogyra, British. Ammoniceras.
Fam. 16.—Skeneidae. Shell depressed, with rounded aperture; cephalic tentacles long. Skenea, British.
Fam. 17.—Choristidae. Shell spiral; four cephalic tentacles; eyes absent; two pedal appendages. Choristes.
Fam. 18.—Assimineidae. Eyes at free extremities of tentacles. Assiminea, estuarine, British.
Fam. 19.—Truncatellidae. Snout very long, bilobed; foot short. Truncatella.
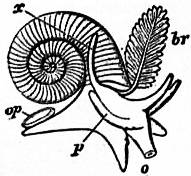 |
| Fig. 30.—Valvata cristata, Müll. |
o, Mouth. op, Operculum. br, Ctenidium (branchial plume). x, Filiform appendage (? rudimentary ctenidium). |
| The freely projecting ctenidium of typical form not having its axis fused to the roof of the branchial chamber is the notable character of this genus. |
Fam. 20.—Hydrobiidae. Shell with prominent spire; penis distant from right tentacle, generally appendiculated; brackish water or fluviatile. Hydrobia, British. Baikalia, from Lake Baikal. Pomatiopsis. Bithynella. Lithoglyphus. Spekia, viviparous, from Lake Tanganyika. Tanganyicia. Limnotrochus, from Lake Tanganyika. Chytra. Littorinida. Bithynia, British, fluviatile. Stenothyra.
Fam. 21.—Melaniidae. Spire of shell somewhat elongated; mantle-border fringed; viviparous; fluviatile. Melania. Faunus. Paludomus. Melanopsis. Nassopsis. Bythoceras, from Lake Tanganyika.
Fam. 22.—Typhobiidae. Foot wide; shell turriculated, with carinated whorls, the carinae tuberculated or spiny. Typhobia. Bathanalia, from Lake Tanganyika.
Fam. 23.—Pleuroceridae. Like Melaniidae, but mantle-border not fringed and reproduction oviparous. Pleurocera. Anculotus.
Fam. 24.—Pseudomelaniidae. All extinct.
Fam. 25.—Subulitidae. All extinct.
Fam. 26.—Nerineidae. All extinct.
Fam. 27.—Cerithiidae. Shell with numerous tuberculated whorls; aperture canaliculated anteriorly; short pallial siphon. Cerithium. Bittium. Potamides. Triforis. Laeocochlis. Cerithiopsis.
Fam. 28.—Modulidae. Shell with short spire; no siphon. Modulus.
Fam. 29.—Vermetidae. Animal fixed by the shell, the last whorls of which are not in contact with each other; foot small; two anterior pedal tentacles. Vermetus. Siliquaria.
Fam. 30.—Caecidae. Shell almost completely uncoiled, in one plane, with internal septa. Caecum, British.
Fam. 31.—Turritellidae. Shell very long; head large; foot broad. Turritella, British. Mesalia. Mathilda.
Fam. 32.—Struthiolariidae. Shell conical; aperture slightly canaliculated; siphon slightly developed. Struthiolaria.
Fam. 33.—Chenopodidae. Shell elongated; aperture expanded; siphon very short. Chenopus, British. Alaria, Spinigera, Diartema, extinct.
Fam. 34.—Strombidae. Foot narrow, compressed, without sole. Strombus. Pteroceras. Rostellaria. Terebellum.
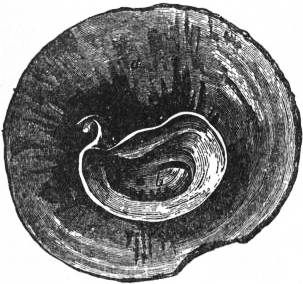 |
| Fig. 31.—Shell of Crucibulum, seen from below so as to show the inner whorl b, concealed by the cap-like outer whorl a. |
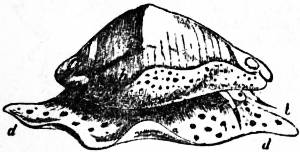 |
| Fig. 32.—Animal and shell of Ovula. |
b, Cephalic tentacles. d, Foot. h, Mantle-skirt, which is naturally carried in a reflected condition so as to cover the sides of the shell. |
Fam. 35.—Xenophoridae. Foot transversely divided into two parts. Xenophorus. Eotrochus, Silurian.
Fam. 36.—Capulidae. Shell conical, not coiled, but slightly incurved posteriorly; a tongue-shaped projection between snout and foot. Capulus. Thyca, parasitic on asterids. Platyceras, extinct.
Fam. 37.—Hipponycidae. Shell conical; foot secreting a ventral calcareous plate; animal fixed. Hipponyx. Mitrularia.
Fam. 38.—Calyptraeidae. Shell with short spire; lateral cervical lobes present; accessory genital glands. Calyptraea, British. Crepidula. Crucibulum.
Fam. 39.—Naricidae. Foot divided into two, posterior half bearing the operculum; a wide epipodial velum; shell turbinated. Narica.
Fam. 40.—Naticidae. Foot large, with aquiferous system; propodium reflected over head; eyes degenerate; burrowing habit. Natica, British. Amaura. Sigaretus.
Fam. 41.—Lamellariidae. Shell thin, more or less covered by the mantle; no operculum. Lamellaria. Velutina. Marsenina, Oncidiopsis, hermaphrodite.
Fam. 42.—Trichotropidae. Shell with short spire, carinate and pointed. Trichotropis.
Fam. 43.—Seguenziidae. Shell trochiform, with canaliculated aperture and twisted columella. Seguenzia, abyssal.
Fam. 44.—Janthinidae. Shell thin; operculum absent; tentacles bifid; foot secretes a float; pelagic. Janthina. Recluzia.
Fam. 45.—Cypraeidae. Shell inrolled, solid, polished, aperture very narrow in adult; short siphon; anus posterior; osphradium with three lobes; mantle reflected over shell. Cypraea. Pustularia. Ovula. Pedicularia, attached to corals. Erato.
Fam. 46.—Tritonidae. Shell turriculated and siphonated, thick, each whorl with varices; foot broad and truncated anteriorly; pallial siphon well developed; proboscis present. Triton. Persona. Ranella.
Fam. 47.—Columbellinidae. All extinct.
Fam. 48.—Cassididae. Shell ventricose, with elongated aperture, and short spire; proboscis and siphon long; operculum with marginal nucleus. Cassis. Cassidaria. Oniscia.
Fam. 49—Oocorythidae. Shell globular and ventricose; aperture oval and canaliculated; operculum spiral. Oocorys, abyssal.
Fam. 50.—Doliidae. Shell ventricose, with short spire, and wide aperture; no varices and no operculum; foot very broad, with projecting anterior angles; siphon long. Dolium. Pyrula.
Fam. 51.—Solariidae. Solarium. Torinia. Fluxina.
Fam. 52.—Scalariidae. Shell turriculated, with elongated spire; proboscis short; siphon rudimentary. Scalaria. Eglisia. Crossea. Aclis.
The three following families have neither radula nor jaws, and are therefore called Aglossa. They have a well-developed proboscis which is used as a suctorial organ; some are abyssal, but the majority are either commensals or parasites of Echinoderms.
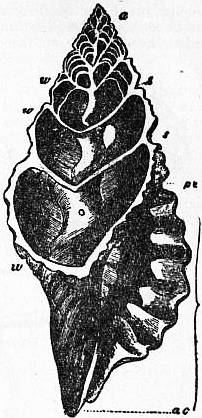 |
| Fig. 33.—Section of the shell of Triton, Cuv. (From Owen.) |
a, Apex. ac, Siphonal notch of the mouth of the shell. ac to pc, Mouth of the shell. w, w, Whorls of the shell. s, s. Sutures. |
| Occupying the axis, and exposed by the section, is seen the “columella” or spiral pillar. The upper whorls of the shell are seen to be divided into separate chambers by the formation of successively formed “septa.” |
Fam. 53.—Pyramidellidae. Summit of spire heterostrophic; a projection, the mentum, between head and foot; operculum present. Pyramidella. Turbonilla. Odostomia, British. Myxa.
Fam. 54.—Eulimidae. Visceral mass still coiled spirally; shell thin and shining. Eulima, foot well developed, with an operculum, animal usually free, but some live in the digestive cavity of Holothurians. Mucronalia, foot reduced, but still operculate, eyes present, animal fixed by its very long proboscis which is deeply buried in the tissues of an Echinoderm, no pseudopallium. Stylifer, the operculum is lost, animal fixed by a large proboscis which forms a pseudopallium covering the whole shell except the extremity of the spire, parasitic on all groups of Echinoderms. Entosiphon, visceral mass still coiled; shell much reduced, proboscis very long forming a pseudopallium which covers the whole body and projects beyond in the form of a siphon, foot and nervous system present, eyes, branchia and anus absent, parasite in the Holothurian Deima blakei in the Indian Ocean.
Fam. 55.—Entoconchidae. No shell; visceral mass not coiled; no sensory organs, nervous system, branchia or anus; body reduced to a more or less tubular sac; hermaphrodite and viviparous; parasitic in Holothurians; larvae are veligers, with shell and operculum. Entocolax, mouth at free extremity, animal fixed by aboral orifice of pseudopallium, Pacific. Entoconcha, body elongated and tubular, animal fixed by the oral extremity, protandric hermaphrodite, parasitic in testes of Holothurians causing their abortion. Enteroxenos, no pseudopallium and no intestine, hermaphrodite, larvae with operculum.
Tribe 2.—Heteropoda. Pelagic Taenioglossa with foot large and laterally compressed to form a fin.
Fam. 1. Atlantidae. Visceral sac and shell coiled in one plane; foot divided transversely into two parts, posterior part bearing an operculum, anterior part forming a fin provided with a sucker. Atlanta. Oxygyrus.
Fam. 2.—Carinariidae. Visceral sac and shell small in proportion to the rest of the body, which cannot be withdrawn into the shell; foot elongated, fin-shaped, with sucker, but without operculum. Carinaria. Cardiopoda.
Fam. 3.—Pterotrachaeidae. Visceral sac very much reduced; without shell or mantle; anus posterior; foot provided with sucker in male only. Pterotrachaea. Firoloida. Pterosoma.
Sub-order 2.—Stenoglossa. Radula narrow with one lateral tooth on each side, and one median tooth or none.
Tribe 1.—Rachiglossa. Radula with a median tooth and a single tooth on each side of it. Formula 1 : 1 : 1. Rudimentary jaws present.
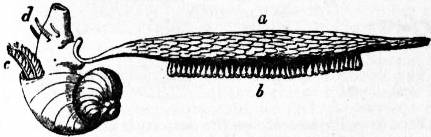 |
| Fig. 34.—Female Janthina, with egg-float (a) attached to the foot; b, egg-capsules; c, ctenidium (gill-plume); d, cephalic tentacles. |
Fam. 1.—Turbinellidae. Shell solid, piriform, with thick folded columella; lateral teeth of radula bicuspidate. Turbinella. Cynodonta. Fulgur. Hemifusus. Tudicla. Strepsidura.
Fam. 2.—Fasciolariidae. Shell elongated, with long siphon; lateral teeth of radula multicuspidate. Fasciolaria. Fusus. Clavella. Latirus.
Fam. 3.—Mitridae. Shell fusiform and solid, aperture elongated, columella folded; no operculum; eyes on sides of tentacles. Mitra. Turricula. Cylindromitra. Imbricaria.
Fam. 4.—Buccinidae. Foot large and broad; eyes at base of tentacles; operculum horny. Buccinum. Chrysodomus. Liomesus. Cominella. Tritonidea. Pisania. Euthria. Phos. Dipsacus.
Fam. 5.—Nassidae. Foot broad, with two slender posterior appendages; operculum unguiculate. Nassa, marine, British. Canidia, fluviatile. Bullia.
Fam. 6.—Muricidae. Shell with moderately long spire and canal, ornamented with ribs, often spiny; foot truncated anteriorly. Murex, British. Trophon, British. Typhis. Urosalpinx. Lachesis.
Fam. 7.—Purpuridae. Shell thick, with short spire, last whorl large and canal short; aperture wide; operculum horny. Purpura, British. Rapana. Monoceros. Sistrum. Concholepas.
Fam. 8.—Haliidae. Shell ventricose, thin and smooth, with wide aperture; foot large and thick, without operculum. Halia.
Fam. 9.—Cancellariidae. Shell ovoid, with short spire and folded columella; foot small, no operculum; siphon short. Cancellaria.
Fam. 10.—Columbellidae. Spire of shell prominent, aperture narrow, canal very short, columella crenelated; foot large. Columbella.
Fam. 11.—Coralliophilidae. Shell irregular; radula absent; foot and siphon short; sedentary animals, living in corals. Coralliophila. Rhizochilus. Leptoconchus. Magilus. Rapa.
Fam. 12.—Volutidae. Head much flattened and wide, with eyes on sides; foot broad; siphon with internal appendages. Valuta. Guivillea. Cymba.
Fam. 13.—Olividae. Foot with anterior transverse groove; a posterior pallial tentacle; generally burrowing. Olivia. Olivella. Ancillaria. Agaronia.
Fam. 14.—Marginellidae. Foot very large; mantle reflected over shell. Marginella. Pseudomarginella.
Fam. 15.—Harpidae. Foot very large; without operculum; shell with short spire and longitudinal ribs; siphon long. Harpa.
Tribe 2.—Toxiglossa. No jaws. No median tooth in radula. Formula: 1 : 0 : 1. Poison-gland present whose duct traverses the nerve-collar.
Fam. 1.—Pleurotomatidae. Shell fusiform, with elongated spire; margin of shell and mantle notched. Pleurotoma. Clavatula. Mangilia. Bela. Pusionella. Pontiothauma.
Fam. 2.—Terebridae. Shell turriculated, with numerous whorls; aperture and operculum oval; eyes at summits of tentacles; siphon long. Terebra.
Fam. 3.—Conidae. Shell conical, with very short spire, and narrow aperture with parallel borders; operculum unguiform Conus.
Sub-Class II.—Euthyneura
The most important general character of the Euthyneura is the absence of torsion in the visceral commissure, and the more posterior position of the anus and pallial organs. Comparative anatomy and embryology prove that this condition is due, not as formerly supposed to a difference in the relations of the visceral commissure which prevented it from being included in the torsion of the visceral hump, but to an actual detorsion which has taken place in evolution and is repeated to a great extent in individual development. In several of the more primitive forms the same torsion occurs as in Streptoneura, viz. in Actaeon and Limacina among Opisthobranchia, and Chilina among Pulmonata. Actaeon is proso-branchiate, the visceral commissure is twisted in Actaeon and Chilina, and even slightly still in Bulla and Scaphander; in Actaeon and Limacina the osphradium is to the left, innervated by the supra-intestinal ganglion. But in the other members of the sub-class the detorsion of the visceral mass has carried back the anus and circumanal complex from the anterior dorsal region to the right side, as in Bulla and Aplysia, or even to the posterior end of the body, as in Philine, Oncidium, Doris, &c. Different degrees of the same process of detorsion are, as we have seen, exhibited by the Heteropoda among the Streptoneura, and both in them and in the Euthyneura the detorsion is associated with degeneration of the shell. Where the modification is carried to its extreme degree, not only the shell but the pallial cavity, ctenidium and visceral hump disappear, and the body acquires a simple elongated form and a secondary external symmetry, as in Pterotrachaea and in Doris, Eolis, and other Nudibranchia. These facts afford strong support to the hypothesis that the weight of the shell is the original cause of the torsion of the dorsal visceral mass in Gastropods. But this hypothesis leaves the elevation of the visceral mass and the exogastric coiling of the shell in the ancestral form unexplained. In those Euthyneura in which the shell is entirely absent in the adult, it is, except in the three genera Cenia, Runcina and Vaginula, developed in the larva and then falls off. In other cases (Tectibranchs) the reduced shell is enclosed by upgrowths of the edge of the mantle and becomes internal, as in many Cephalopods. A few Euthyneura in which the shell is not much reduced retain an operculum in the adult state, e.g. Actaeon, Limacina, and the marine Pulmonate, Amphibola. The detorted visceral commissure shows a tendency to the concentration of all its elements round the oesophagus, so that except in the Bullomorpha and in Aplysia the whole nervous system is aggregated in the cephalic region, either dorsally or ventrally. The radula has a number of uniform teeth on each side of the median tooth in each transverse row. The head in most cases bears two pairs of tentacles. All the Euthyneura are hermaphrodite.
 |
| Fig. 35.—Acera bullata. A single row of teeth of the Radula. (Formula, x.l.x.) |
In the most primitive condition the genital duct is single throughout its length and has a single external aperture; it is therefore said to be monaulic. The hermaphrodite aperture is on the right side near the opening of the pallial cavity, and a ciliated groove conducts the spermatozoa to the penis, which is situated more anteriorly. This is the condition in the Bullomorpha, the Aplysiomorpha, and in one Pulmonate, Pythia. In some cases while the original aperture remains undivided, the seminal groove is closed and so converted into a canal. This is the modification found in Cavolinia longirostris among the Bullomorpha, and in all the Auriculidae except Pythia. A further degree of modification occurs when the male duct takes its origin from the hermaphrodite duct above the external opening, so that there are two distinct apertures, one male and one female, the latter being the original opening. The genital duct is now said to be diaulic, as in Valvata, Oncidiopsis, Actaeon, and Lobiger among the Bullomorpha, in the Pleurobranchidae, in the Nudibranchia, except the Doridomorpha and most of the Elysiomorpha, and in the Pulmonata. Originally in this condition the female aperture is at some distance from the male, as in the Basommatophora and in other cases; but in some forms the female aperture itself has shifted and come to be contiguous with the male opening and penis as in the Stylommatophora. In all these cases the female duct bears a bursa copulatrix or receptaculum seminis. In some forms this receptacle acquires a separate external opening remaining connected with the oviduct internally. There are thus two female openings, one for copulation, the other for oviposition, as well as a male opening. The genital duct is now trifurcated or triaulic, a condition which is confined to certain Nudibranchs, viz. the Doridomorpha and most of the Elysiomorpha.
The Pteropoda, formerly regarded as a distinct class of the Mollusca, were interpreted by E.R. Lankester as a branch of the Cephalopoda, chiefly on account of the protrusible sucker-bearing processes at the anterior end of Pneumonoderma. These he considered to be homologous with the arms of Cephalopods. He fully recognized, however, the similarity of Pteropods to Gastropods in their general asymmetry and in the torsion of the visceral mass in Limacinidae. It is now understood that they are Euthyneurous Gastropods adapted to natatory locomotion and pelagic life. The sucker-bearing processes of Pneumonoderma are outgrowths of the proboscis. The fins of Pteropods are now interpreted as the expanded lateral margins of the foot, termed parapodia, not homologous with the siphon of Cephalopods which is formed from epipodia. The Thecosomatous Pteropoda are allied to Bulla, the Gymnosomatous forms to Aplysia. The Euthyneura comprises two orders, Opisthobranchia and Pulmonata.
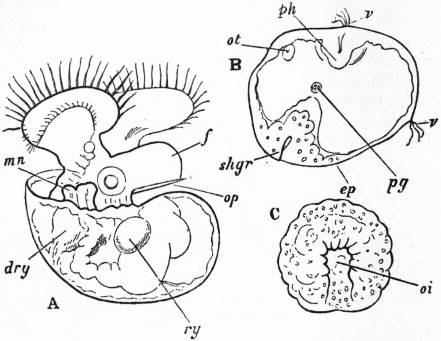 |
| Fig. 36. |
A, Veliger-larva of an Opisthobranch (Polycera). f, Foot; op, operculum; mn, anal papilla; ry, dry, two portions of unabsorbed nutritive yolk on either side of the intestine. The right otocyst is seen at the root of the foot.
B, Trochosphere of an Opisthobranch (Pleurobranchidium) showing—shgr, the shell-gland or primitive shell-sac; v, the cilia of the velum; ph, the commencing stomodaeum or oral invagination; ot, the left otocyst; pg, red-coloured pigment spot.
C, Diblastula of an Opisthobranch (Polycera) with elongated blastopore oi.
(All from Lankester.)
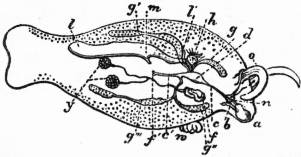 |
| Fig. 37.—Phyllirhoë bucephala, twice the natural size, a transparent pisciform pelagic Opisthobranch. The internal organs are shown as seen by transmitted light. (After W. Keferstein.) |
a, Mouth. b, Radular sac. c, Oesophagus. d, Stomach. c’, Intestine. f’, Anus. g, g′, g″, g″′, The four lobes of the liver. h, The heart (auricle and ventricle). l, The renal sac (nephridium). l′, The ciliated communication of the renal sac with the pericardium. m, The external opening of the renal sac. n, The cerebral ganglion. o, The cephalic tentacles. f, The genital pore. y, The ovo-testes. w, The parasitic hydromedusa Mnestra, usually found attached in this position by the aboral pole of its umbrella. |
Order 1.—Opisthobranchia. Marine Euthyneura, the more archaic forms of which have a relatively large foot and a small visceral hump, from the base of which projects on the right side a short mantle-skirt. The anus is placed in such forms far back beyond the mantle-skirt. In front of the anus, and only partially covered by the mantle-skirt, is the ctenidium with its free end turned backwards. The heart lies in front of, instead of to the side of, the attachment of the ctenidium—hence Opisthobranchia as opposed to “Prosobranchia,” which correspond to the Streptoneura. A shell is possessed in the adult state by but few Opisthobranchia, but all pass through a veliger larval stage with a nautiloid shell (fig. 36). Many Opisthobranchia have by a process of atrophy lost the typical ctenidium and the mantle-skirt, and have developed other organs in their place. As in some Pectinibranchia, the free margin of the mantle-skirt is frequently reflected over the shell when a shell exists; and, as in some Pectinibranchia, broad lateral outgrowths of the foot (parapodia) are often developed which may be thrown over the shell or naked dorsal surface of the body.
The variety of special developments of structure accompanying the atrophy of typical organs in the Opisthobranchia and general degeneration of organization is very great. The members of the order present the same wide range of superficial appearance as do the Pectinibranchiate Streptoneura, forms carrying well-developed spiral shells and large mantle-skirts being included in the group, together with flattened or cylindrical slug-like forms. But in respect of the substitution of other parts for the mantle-skirt and for the gill which the more degenerate Opisthobranchia exhibit, this order stands alone. Some Opisthobranchia are striking examples of degeneration (some Nudibranchia), having none of those regions or processes of the body developed which distinguish the archaic Mollusca from such flat-worms as the Dendrocoel Planarians. Indeed, were it not for their retention of the characteristic odontophore we should have little or no indication that such forms as Phyllirhoë and Limapontia really belong to the Mollusca at all. The interesting little Rhodope veranyii, which has no odontophore, has been associated by systematists both with these simplified Opisthobranchs and with Rhabdocoel Planarians.
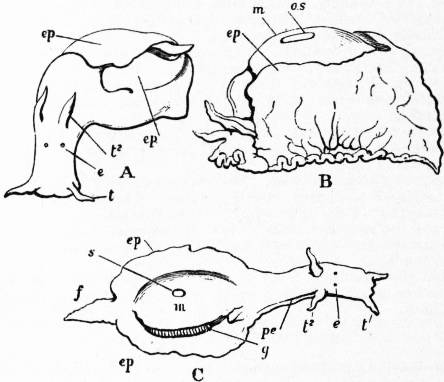 |
|
| Fig. 38.—Three views of Aplysia sp., in various conditions of expansion and retraction. (After Cuvier.) | |
t, Anterior cephalic tentacles. t², Posterior cephalic tentacles. e, Eyes. f, Metapodium. ep, Epipodium. |
g, Gill-plume (ctenidium). m, Mantle-flap reflected over the thin oval shell. os, s, Orifice formed by the unclosed border of the reflected mantle-skirt, allowing the shell to show. pe, The spermatic groove. |
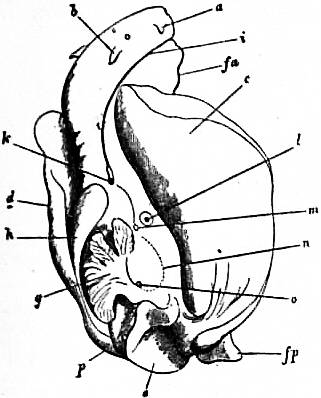 |
| Fig. 39.—Aplysia leporina (camelus, Cuv.), with epipodia and mantle reflected away from the mid-line. (Lankester.) |
a, Anterior cephalic tentacle. b, Posterior cephalic tentacle; between a and b, the eyes. c, Right epipodium. d, Left epipodium. e, Hinder part of visceral hump. fp, Posterior extremity of the foot. fa, Anterior part of the foot underlying the head. g, The ctenidium (branchial plume). h, The mantle-skirt tightly spread over the horny shell and pushed with it towards the left side. i, The spermatic groove. k, The common genital pore (male and female). l, Orifice of the grape-shaped (supposed poisonous) gland. m, The osphradium (olfactory organ of Spengel). n, Outline of part of the renal sac (nephridium) below the surface. o, External aperture of the nephridium. p, Anus. |
In many respects the sea-hare (Aplysia), of which several species are known (some occurring on the English coast), serves as a convenient example of the fullest development of the organization characteristic of Opisthobranchia. The woodcut (fig. 38) gives a faithful representation of the great mobility of the various parts of the body. The head is well marked and joined to the body by a somewhat constricted neck. It carries two pairs of cephalic tentacles and a pair of sessile eyes. The visceral hump is low and not drawn out into a spire. The foot is long, carrying the oblong visceral mass upon it, and projecting (as metapodium) a little beyond it (f). Laterally the foot gives rise to a pair of mobile fleshy lobes, the parapodia (ep), which can be thrown up so as to cover in the dorsal surface of the animal. Such parapodia are common, though by no means universal, among Opisthobranchia. The torsion of the visceral hump is not carried out very fully, the consequence being that the anus has a posterior position a little to the right of the median line above the metapodium, whilst the branchial chamber formed by the overhanging mantle-skirt faces the right side of the body instead of lying well to the front as in Streptoneura and as in Pulmonate Euthyneura. The gill-plume, which in Aplysia is the typical Molluscan ctenidium, is seen in fig. 39 projecting from the branchial sub-pallial space. The relation of the delicate shell to the mantle is peculiar, since it occupies an oval area upon the visceral hump, the extent of which is indicated in fig. 38, C, but may be better understood by a glance at the figures of the allied genus Umbrella (fig. 40), in which the margin of the mantle-skirt coincides, just as it does in the limpet, with the margin of the shell. But in Aplysia the mantle is reflected over the edge of the shell, and grows over its upper surface so as to completely enclose it, excepting at the small central area s where the naked shell is exposed. This enclosure of the shell is a permanent development of the arrangement seen in many Streptoneura (e.g. Pyrula, Ovula, see figs. 18 and 32), where the border of the mantle can be, and usually is, drawn over the shell, though it is withdrawn (as it cannot be in Aplysia) when they are irritated. From the fact that Aplysia commences its life as a free-swimming veliger with a nautiloid shell not enclosed in any way by the border of the mantle, it is clear that the enclosure of the shell in the adult is a secondary process. Accordingly, the shell of Aplysia must not be confounded with a primitive shell in its shell-sac, such as we find realized in the shells of Chiton and in the plugs which form in the remarkable transitory “shell-sac” or “shell-gland” of Molluscan embryos (see figs. 26, 60). Aplysia, like other Mollusca, develops a primitive shell-sac in its trochosphere stage of development, which disappears and is succeeded by a nautiloid shell (fig. 36). This forms the nucleus of the adult shell, and, as the animal grows, becomes enclosed by a reflection of the mantle-skirt. When the shell of an Aplysia enclosed in its mantle is pushed well to the left, the sub-pallial space is fully exposed as in fig. 39, and the various apertures of the body are seen. Posteriorly we have the anus, in front of this the lobate gill-plume, between the two (hence corresponding in position to that of the Pectinibranchia) we have the aperture of the renal organ. In front, near the anterior attachment of the gill-plume, is the osphradium (olfactory organ) discovered by J.W. Spengel, yellowish in colour, in the typical position, and overlying an olfactory ganglion with typical nerve-connexion (see fig. 43). To the right of Spengel’s osphradium is the opening of a peculiar gland which has, when dissected out, the form of a bunch of grapes; its secretion is said to be poisonous. On the under side of the free edge of the mantle are situated the numerous small cutaneous glands which, in the large Aplysia camelus (not in other species), form the purple secretion which was known to the ancients. In front of the osphradium is the single genital pore, the aperture of the common or hermaphrodite duct. From this point there passes forward to the right side of the head a groove—the spermatic groove—down which the spermatic fluid passes. In other Euthyneura this groove may close up and form a canal. At its termination by the side of the head is the muscular introverted penis. In the hinder part of the foot (not shown in any of the diagrams) is the opening of a large mucus-forming gland very often found in the Molluscan foot.
With regard to internal organization we may commence with the disposition of the renal organ (nephridium), the external opening of which has already been noted. The position of this opening and other features of the renal organ were determined by J.T. Cunningham.
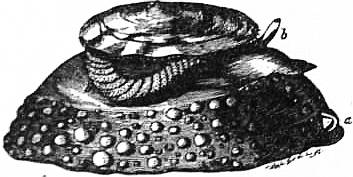 |
| Fig. 40.—Umbrella mediterranea. a, mouth; b, cephalic tentacle; h, gill (ctenidium). The free edge of the mantle is seen just below the margin of the shell (compare with Aplysia, fig. 39). (From Owen.) |
There is considerable uncertainty with respect to the names of the species of Aplysia. There are two forms which are very common in the Gulf of Naples. One is quite black in colour, and measures when outstretched 8 or 9 in. in length. The other is light brown and somewhat smaller, its length usually not exceeding 7 in. The first is flaccid and sluggish in its movements, and has not much power of contraction; its epipodial lobes are enormously developed and extend far forward along the body; it gives out when handled an abundance of purple liquid, which is derived from cutaneous glands situated on the under side of the free edge of the mantle. According to F. Blochmann it is identical with A. camelus of Cuvier. The other species is A. depilans; it is firm to the touch, and contracts forcibly when irritated; the secretion of the mantle-glands is not abundant, and is milky white in appearance. The kidney has similar relations in both species, and is identical with the organ spoken of by many authors as the triangular gland. Its superficial extent is seen when the folds covering the shell are cut away and the shell removed; the external surface forms a triangle with its base bordering the pericardium, and its apex directed posteriorly and reaching the the left-hand posterior corner of the shell-chamber. The dorsal surface of the kidney extends to the left beyond the shell-chamber beneath the skin in the space between the shell-chamber and the left parapodium.
When the animal is turned on its left-hand side and the mantle-chamber widely opened, the gill being turned over to the left, a part of the kidney is seen beneath the skin between the attachment of the gill and the right parapodium (fig. 39). On examination this is found to be the under surface of the posterior limb of the gland, the upper surface of which has just been described as lying beneath the shell. In the posterior third of this portion, close to that edge which is adjacent to the base of the gill, is the external opening (fig. 39, o).
When the pericardium is cut open from above in an animal otherwise entire, the anterior face of the kidney is seen forming the posterior wall of the pericardial chamber; on the deep edge of this face, a little to the left of the attachment of the auricle to the floor of the pericardium, is seen a depression; this depression contains the opening from the pericardium into the kidney.
To complete the account of the relations of the organ: the right anterior corner can be seen superficially in the wall of the mantle-chamber above the gill. Thus the base of the gill passes in a slanting direction across the right-hand side of the kidney, the posterior end being dorsal to the apex of the gland, and the anterior end ventral to the right-hand corner.
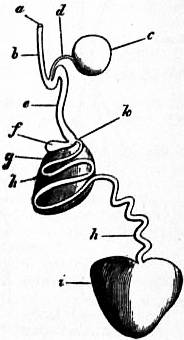 |
| Fig. 41.—Gonad, and accessory glands and ducts of Aplysia. (Lankester.) |
i, Ovo-testis. h, Hermaphrodite duct. g, Albuminiparous gland. f, Vesicula seminalis. k, Opening of the albuminiparous gland into the hermaphrodite duct. e, Hermaphrodite duct (uterine portion). b, Vaginal portion of the uterine duct. c, Spermatheca. d, Its duct. a, Genital pore. |
As so great a part of the whole surface of the kidney lies adjacent to external surfaces of the body, the remaining part which faces the internal organs is small; it consists of the left part of the under surface; it is level with the floor of the pericardium, and lies over the globular mass formed by the liver and convoluted intestine.
Thus the renal organ of Aplysia is shown to conform to the Molluscan type. The heart lying within the adjacent pericardium has the usual form, a single auricle and ventricle. The vascular system is not extensive, the arteries soon ending in the well-marked spongy tissue which builds up the muscular foot, parapodia, and dorsal body-wall.
The alimentary canal commences with the usual buccal mass; the lips are cartilaginous, but not armed with horny jaws, though these are common in other Opisthobranchs; the lingual ribbon is multidenticulate, and a pair of salivary glands pour in their secretion. The oesophagus expands into a curious gizzard, which is armed internally with large horny processes, some broad and thick, others spinous, fitted to act as crushing instruments. From this we pass to a stomach and a coil of intestine embedded in the lobes of a voluminous liver; a caecum of large size is given off near the commencement of the intestine. The liver opens by two ducts into the digestive tract.
The generative organs lie close to the coil of intestine and liver, a little to the left side. When dissected out they appear as represented in fig. 41. The essential reproductive organ or gonad consists of both ovarian and testicular cells (see fig. 42). It is an ovo-testis. From it passes a common or hermaphrodite duct, which very soon becomes entwined in the spire of a gland—the albuminiparous gland. The latter opens into the common duct at the point k, and here also is a small diverticulum of the duct f. Passing on, we find not far from the genital pore a glandular spherical body (the spermatheca c) opening by means of a longish duct into the common duct, and then we reach the pore (fig. 39, k). Here the female apparatus terminates. But when the male secretion of the ovo-testis is active, the seminal fluid passes from the genital pore along the spermatic groove (fig. 39) to the penis, and is by the aid of that eversible muscular organ introduced into the genital pore of a second Aplysia, whence it passes into the spermatheca, there to await the activity of the female element of the ovo-testis of this second Aplysia. After an interval of some days—possibly weeks—the ova of the second Aplysia commence to descend the hermaphrodite duct; they become enclosed in a viscid secretion at the point where the albuminiparous gland opens into the duct intertwined with it; and on reaching the point where the spermathecal duct debouches they are impregnated by the spermatozoa which escape now from the spermatheca and meet the ova.
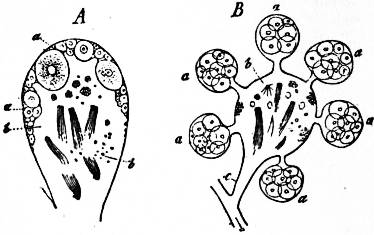 |
| Fig. 42.—Follicles of the hermaphrodite gonads of Euthyneurous Gastropods. A, of Helix; B, of Eolis; a, ova; b, developing spermatozoa; c, common efferent duct. |
 |
| Fig. 43.—Nervous system of Aplysia, as a type of the long-looped Euthyneurous condition. The untwisted visceral loop is lightly shaded. (After Spengel.) |
ce, Cerebral ganglion. pl, Pleural ganglion. pe, Pedal ganglion. ab. sp, Abdominal ganglion which represents also the supra-intestinal ganglion of Streptoneura and gives off the nerve to the osphradium (olfactory organ) o, and another to an unlettered so-called “genital” ganglion. The buccal nerves and ganglia are omitted. |
The development of Aplysia from the egg presents many points of interest from the point of view of comparative embryology, but in relation to the morphology of the Opisthobranchia it is sufficient to point to the occurrence of a trochosphere and a veliger stage (fig. 36), and of a shell-gland or primitive shell-sac (fig. 36, shgr), which is succeeded by a nautiloid shell.
In the nervous system of Aplysia the great ganglion-pairs are well developed and distinct. The euthyneurous visceral loop is long, and presents only one ganglion (in Aplysia camelus, but two distinct ganglia joined to one another in Aplysia hybrida of the English coast), placed at its extreme limit, representing both the right and left visceral ganglia and the third or abdominal ganglion, which are so often separately present. The diagram (fig. 43) shows the nerve connecting this abdomino-visceral ganglion with the olfactory ganglion of Spengel. It is also seen to be connected with a more remote ganglion—the genital. Such special irregularities in the development of ganglia upon the visceral loop, and on one or more of the main nerves connected with it, are very frequent. Our figure of the nervous system of Aplysia does not give the small pair of buccal ganglia which are, as in all glossophorous Molluscs, present upon the nerves passing from the cerebral region to the odontophore.
For a comparison of various Opisthobranchs, Aplysia will be found to present a convenient starting-point. It is one of the more typical Opisthobranchs, that is to say, it belongs to the section Tectibranchia, but other members of the suborder, namely, Bulla and Actaeon (figs. 44 and 45), are less abnormal than Aplysia in regard to their shells and the form of the visceral hump. They have naked spirally twisted shells which may be concealed from view in the living animal by the expansion and reflection of the parapodia, but are not enclosed by the mantle, whilst Actaeon is remarkable for possessing an operculum like that of so many Streptoneura.
The great development of the parapodia seen in Aplysia is usual in Tectibranchiate Opisthobranchs. The whole surface of the body becomes greatly modified in those Nudibranchiate forms which have lost, not only the shell, but also the ctenidium. Many of these have peculiar processes developed on the dorsal surface (fig. 46, A, B), or retain purely negative characters (fig. 46, D). The chief modification of internal organization presented by these forms, as compared with Aplysia, is found in the condition of the alimentary canal. The liver is no longer a compact organ opening by a pair of ducts into the median digestive tract, but we find very numerous hepatic diverticula on a shortened axial tract (fig. 47). These diverticula extend usually one into each of the dorsal papillae or “cerata” when these are present. They are not merely digestive glands, but are sufficiently wide to act as receptacles of food, and in them the digestion of food proceeds just as in the axial portion of the canal. A precisely similar modification of the liver or great digestive gland is found in the scorpions, where the axial portion of the digestive canal is short and straight, and the lateral ducts sufficiently wide to admit food into the ramifications of the gland there to be digested; whilst in the spiders the gland is reduced to a series of simple caeca.
 |
| Fig. 44.—Bulla vexillum (Chemnitz), as seen crawling. á, oral hood (compare with Tethys, fig. 46, B), possibly a continuation of the epipodia; b, b′, cephalic tentacles. (From Owen.) |
The typical character is retained by the heart, pericardium, and the communicating nephridium or renal organ in all Opisthobranchs. An interesting example of this is furnished by the fish-like transparent Phyllirhoë (fig. 37), in which it is possible most satisfactorily to study in the living animal, by means of the microscope, the course of the blood-stream, and also the reno-pericardial communication. In many of the Nudibranchiate Opisthobranchs the nervous system presents a concentration of the ganglia (fig. 48), contrasting greatly with what we have seen in Aplysia. Not only are the pleural ganglia fused to the cerebral, but also the visceral to these (see in further illustration the condition attained by the Pulmonate Limnaeus, fig. 59), and the visceral loop is astonishingly short and insignificant (fig. 48, e′). That the parts are rightly thus identified is probable from J.W. Spengel’s observation of the osphradium and its nerve-supply in these forms; the nerve to that organ, which is placed somewhat anteriorly—on the dorsal surface—being given off from the hinder part (visceral) of the right compound ganglion—the fellow to that marked A in fig. 48. The Eolid-like Nudibranchs, amongst other specialities of structure, possess (in some cases at any rate) apertures at the apices of the “cerata” or dorsal papillae, which lead from the exterior into the hepatic caeca. Some amongst them (Tergipes, Eolis) are also remarkable for possessing peculiarly modified cells placed in sacs (cnidosacs) at the apices of these same papillae, which resemble the “thread-cells” of the Coelentera. According to T.S. Wright and J.H. Grosvenor these nematocysts are derived from the hydroids on which the animals feed.
 |
| Fig. 45.—Actaeon. h, shell; b, oral hood; d, foot; f, operculum. |
The development of many Opisthobranchia has been examined—e.g. Aplysia, Pleurobranchidium, Elysia, Polycera, Doris, Tergipes. All pass through trochosphere and veliger stages, and in all a nautiloid or boat-like shell is developed, preceded by a well-marked “shell-gland” (see fig. 36). The transition from the free-swimming veliger larva with its nautiloid shell (fig. 36) to the adult form has not been properly observed, and many interesting points as to the true nature of folds (whether parapodia or mantle or velum) have yet to be cleared up by a knowledge of such development in forms like Tethys, Doris, Phyllidia, &c. As in other Molluscan groups, we find even in closely-allied genera (for instance, in Aplysia and Pleurobranchidium, and other genera), the greatest differences as to the amount of food-material by which the egg-shell is encumbered. Some form their diblastula by emboly, others by epiboly; and in the later history of the further development of the enclosed cells (arch-enteron) very marked variations occur in closely-allied forms, due to the influence of a greater or less abundance of food-material mixed with the protoplasm of the egg.
Sub-order 1.—Tectibranchia. Opisthobranchs provided in the adult state with a shell and a mantle, except Runcina, Pleurobranchaea, Cymbuliidae, and some Aplysiomorpha. There is a ctenidium, except in some Thecosomata and Gymnosomata, and an osphradium.
Tribe 1.—Bullomorpha. The shell is usually well developed, except in Runcina and Cymbuliidae, and may be external or internal. No operculum, except in Actaeonidae and Limacinidae. The pallial cavity is always well developed, and contains the ctenidium, at least in part; ctenidium, except in Lophocercidae, of folded type. With the exception of the Aplustridae, Lophocercidae and Thecosomata, the head is devoid of tentacles, and its dorsal surface forms a digging disk or shield. The edges of the foot form parapodia, often transformed into fins. Posteriorly the mantle forms a large pallial lobe under the pallial aperture. Stomach generally provided with chitinous or calcified masticatory plates. Visceral commissure fairly long, except in Runcina, Lobiger and Thecosomata. Hermaphrodite genital aperture, connected with the penis by a ciliated groove, except in Actaeon, Lobiger and Cavolinia longirostris, in which the spermiduct is a closed tube. Animals either swim or burrow.
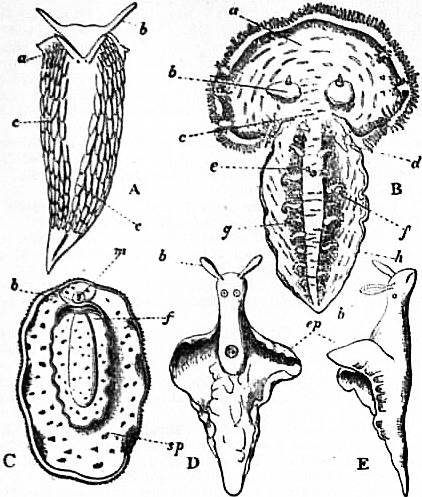 |
|
| Fig. 46. | |
| A, Eolis papillosa (Lin.), dorsal view. | |
a, b, Posterior and anterior cephalic tentacles. |
c, The dorsal “cerata.” |
| B, Tethys leporina, dorsal view. | |
a, The cephalic hood. b, Cephalic tentacles. c, Neck. d, Genital pore. |
e, Anus. f, Large cerata. g, Smaller cerata. h, Margin of the foot. |
| C, Doris (Actinocyclus) tuberculatus (Cuv.), seen from the pedal surface. | |
m, Mouth. b, Margin of the head. |
f, Sole of the foot. sp, The mantle-like epipodium. |
| D, E, Dorsal and lateral view of Elysia (Actaeon) viridis. ep, epipodial outgrowths. (After Keferstein.) | |
|
|
 |
| Fig. 49.—Cavolinia tridentata, Forsk. from the Mediterranean, magnified two diameters. (From Owen.) |
a, Mouth. b, Pair of cephalic tentacles. C, C, Pteropodial lobes of the foot. d, Median web connecting these. e, e, Processes of the mantle-skirt reflected over the surface of the shell. g, The shell enclosing the visceral hump. h. The median spine of the shell. |
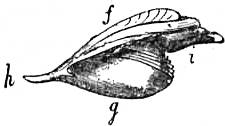 |
| Fig. 50.—Shell of Cavolinia tridentata, seen from the side. |
f, Postero-dorsal surface. g, Antero-ventral surface. h, Median dorsal spine. i, Mouth of the shell. |
Fam. 1.—Actaeonidae. Cephalic shield bifid posteriorly; margins of foot slightly developed; genital duct diaulic; visceral commissure streptoneurous; shell thick, with prominent spire and elongated aperture; a horny operculum. Actaeon, British. Solidula. Tornatellaea, extinct. Adelactaeon. Bullina. Bullinula.
Fam. 2.—Ringiculidae. Cephalic disk enlarged anteriorly, forming an open tube posteriorly; shell external, thick, with prominent spire; no operculum. Ringicula. Pugnus.
Fam. 3.—Tornatinidae. Margins of foot not prominent; no radula; shell external, with inconspicuous spire. Tornatina, British. Retusa. Volvula.
Fam. 4.—Scaphandridae. Cephalic shield short, truncated posteriorly; eyes deeply embedded; three calcareous stomachal plates; shell external, with reduced spire. Scaphander, British. Atys. Smaragdinella. Cylichna, British. Amphisphyra, British.
Fam. 5.—Bullidae. Margins of foot well developed; eyes superficial; three chitinous stomachal plates; shell external, with reduced spire. Bulla, British. Haminea, British.
Fam. 6.—Aceratidae. Cephalic shield continuous with neck; twelve to fourteen stomachal plates; a posterior pallial filament passing through a notch in shell. Acera, British. Cylindrobulla. Volutella.
Fam. 7.—Aplustridae. Foot very broad; cephalic shield with four tentacles; shell external, thin, without prominent spire. Aplustrum. Hydatina. Micromelo.
Fam. 8.—Philinidae. Cephalic shield broad, thick and simple; shell wholly internal, thin, spire much reduced, aperture very large. Philine, British. Cryptophthalmus. Chelinodura. Phanerophthalmus. Colpodaspis, British. Colobocephalus.
Fam. 9.—Doridiidae. Cephalic shield ending posteriorly in a median point; shell internal, largely membranous; no radula or stomachal plates. Doridium. Navarchus.
Fam. 10.—Gastropteridae. Cephalic shield pointed behind; shell internal, chiefly membranous, with calcified nucleus, nautiloid; parapodia forming fins. Gastropteron.
Fam. 11.—Runcinidae. Cephalic shield continuous with dorsal integument; no shell; ctenidium projecting from mantle cavity. Runcina.
Fam. 12.—Lophocercidae. Shell external, globular or ovoid; foot elongated, parapodia separate from ventral surface; genital duct diaulic. Lobiger. Lophocercus.
The next three families form the group formerly known as Thecosomatous Pteropods. They are all pelagic, the foot being entirely transformed into a pair of anterior fins; eyes are absent, and the nerve centres are concentrated on the ventral side of the oesophagus.
Fam. 13.—Limacinidae. Dextral animals, with shell coiled pseudo-sinistrally; operculum with sinistral spiral; pallial cavity dorsal. Limacina, British. Peraclis, ctenidium present.
Fam. 14.—Cymbuliidae. Adult without shell; a sub-epithelial pseudoconch formed by connective tissue; pallial cavity ventral. Cymbulia. Cymbuliopsis. Gleba. Desmopterus.
Fam. 15.—Cavoliniidae. Shell not coiled, symmetrical; pallial cavity ventral. Cavolinia. Clio. Cuvierina.
Tribe 2.—Aplysiomorpha. Shell more or less internal, much reduced or absent. Head bears two pairs of tentacles. Parapodia separate from ventral surface, and generally transformed into swimming lobes. Visceral commissure much shortened, except in Aplysia. Genital duct monaulic; hermaphrodite duct connected with penis by a ciliated groove. Animals either swim or crawl.
Fam. 1.—Aplysiidae. Shell partly or wholly internal, or absent; foot long, with well-developed ventral surface. Aplysia. Dolabella. Dolabrifer. Aplysiella. Phyllaplysia. Notarchus.
The next six families include the animals formerly known as Gymnosomatous Pteropods, characterized by the absence of mantle and shell, the reduction of the ventral surface of the foot, and the parapodial fins at the anterior end of the body. They are all pelagic.
Fam. 2.—Pneumonodermatidae. Pharynx evaginable, with suckers. Pneumonoderma. Dexiobranchaea. Spongiobranchaea. Schizobrachium.
Fam. 3.—Clionopsidae. No buccal appendages or suckers; a very long evaginable proboscis; a quadriradiate terminal branchia. Clionopsis.
Fam. 4.—Notobranchaeidae. Posterior branchia triradiate. Notobranchaea.
Fam. 5.—Thliptodontidae. Head very large, not marked off from the body; neither branchia nor suckers; fins situated near the middle of the body. Thliptodon.
|
|
Fam. 6.—Clionidae. No branchia of any kind; a short evaginable pharynx, bearing paired conical buccal appendages or “cephalocones.” Clione. Paraclione. Fowlerina.
Fam. 7.—Halopsychidae. No branchia; two long and branched buccal appendages. Halopsyche.
Tribe 3.—Pleurobranchomorpha. Two pairs of tentacles. Foot without parapodia; no pallial cavity, but always a single ctenidium situated on the right side between mantle and foot. Genital duct diaulic, without open seminal groove; male and female apertures contiguous. Visceral commissure short, tendency to concentration of all ganglia in dorsal side of oesophagus.
Fam. 1.—Tylodinidae. Shell external and conical; anterior tentacles form a frontal veil; ctenidium extending only over right side; a distinct osphradium. Tylodina.
Fam. 2.—Umbrellidae. Shell external, conical, much flattened; anterior tentacles very small, and situated with the mouth in a notch of the foot below the head; ctenidium very large. Umbrella.
Fam. 3.—Pleurobranchidae. Shell covered by mantle, or absent; anterior tentacles form a frontal veil; mantle contains spicules. Pleurobranchus. Berthella. Haliotinella. Oscanius, British. Oscaniella. Oscaniopsis. Pleurobranchaea.
Sub-order 2.—Nudibranchia. Shell absent in the adult; no ctenidium or osphradium. Body generally slug-like, and externally symmetrical. Visceral mass not marked off from the foot, except in Hedylidae. Dorsal respiratory appendages frequently present. Visceral commissure reduced; nervous system concentrated on dorsal side of oesophagus. Marine; generally carnivorous, and brightly coloured, affording many instances of protective resemblance.
Tribe 1.—Tritoniomorpha. Liver wholly or partially contained in the visceral mass. Anus lateral, on the right side. Usually two rows of ramified dorsal appendages. Genital duct diaulic; male and female apertures contiguous.
Fam. 1.—Tritoniidae. Anterior tentacles form a frontal veil; foot rather broad. Tritonia, British. Marionia.
Fam. 2.—Scyllaeidae. No anterior tentacles; dorsal appendages broad and foliaceous; foot very narrow; stomach with horny plates. Scyllaea, pelagic.
Fam. 3.—Phyllirhoidae. No anterior tentacles, and no dorsal appendages; body laterally compressed, transparent; pelagic. Phyllirhoë.
Fam. 4.—Tethyidae. Head broad, surrounded by a funnel-shaped velum or hood; no radula; dorsal appendages foliaceous. Tethys. Melibe.
Fam. 5.—Dendronotidae. Anterior tentacles forming a scalloped frontal veil; dorsal appendages and tentacles similarly ramified. Dendronotus. Campaspe.
Fam. 6.—Bornellidae. Dorsum furnished on either side with papillae, at the base of which are ramified appendages. Bornella.
Fam. 7.—Lomanotidae. Body flattened, the two dorsal borders prominent and foliaceous. Lomanotus, British.
Tribe 2.—Doridomorpha. Body externally symmetrical; anus median, posterior, and generally dorsal, surrounded by ramified pallial appendages, constituting a secondary branchia. Liver not ramified in the integuments. Genital duct triaulic. Spicules present in the mantle.
|
|
Fam. 1.—Polyceratidae. A more or less prominent frontal veil; branchiae non-retractile. Euplocamus. Polycera, British. Thecacera, British. Aegirus, British. Plocamopherus. Palio. Crimora. Triopa, British. Triopella.
Fam. 2.—Goniodorididae. Mantle-border projecting; frontal veil reduced, and often covered by the anterior border of the mantle. Goniodoris, British. Acanthodoris, British. Idalia, British. Ancula, British. Doridunculus. Lamellidoris. Ancylodoris, the only fresh-water Nudibranch, from Lake Baikal.
Fam. 3.—Heterodorididae. No branchia. Heterodoris.
Fam. 4.—Dorididae. Mantle oval, covering the head and the greater part of the body; anterior tentacles, ill-developed; branchiae generally retractile. Doris, British. Hexabranchus. Chromodoris.
Fam. 5.—Doridopsidae. Pharynx suctorial; no radula; branchial rosette on the dorsal surface, above the mantle-border. Doridopsis.
Fam. 6.—Corambidae. Anus and branchia posterior, below the mantle-border. Corambe.
Fam. 7.--Phyllidiidae. Pharynx suctorial; branchiae surrounding the body, between the mantle and foot. Phyllidia. Fryeria.
The last three families constitute the sub-tribe Porostomata, characterized by the reduction of the buccal mass, which is modified into a suctorial apparatus.
Tribe 3.—Eolidomorpha (Cladohepatica). The whole of the liver contained in the integuments and tegumentary papillae. Genital duct diaulic; male and female apertures contiguous. The anus is antero-lateral, except in the Proctonotidae, in which it is median. Tegumentary papillae not ramified, and containing cnidosacs with nematocysts.
Fam. 1.—Eolididae. Dorsal papillae spindle-shaped or club-shaped. Eolis, British. Facelina, British. Tergipes, British. Gonieolis. Cuthona. Embletonia. Galvina. Calma. Hero.
Fam. 2.—Glaucidae. Body furnished with three pairs of lateral lobes, bearing the tegumentary papillae; foot very narrow; pelagic. Glaucus.
Fam. 3.—Hedylidae. Body elongated; visceral mass marked off from foot posteriorly; dorsal appendages absent, or reduced to a single pair; spicules in the integument. Hedyle.
Fam. 4.—Pseudovermidae. Head without tentacles; body elongated; anus on right side. Pseudovermis.
Fam. 5.—Proctonotidae. Anus posterior, median; anterior tentacles, atrophied; foot broad. Janus, British. Proctonotus, British.
Fam. 6.—Dotonidae. Bases of the rhinophores surrounded by a sheath; dorsal papillae tuberculated and club-shaped, in a single row on either side of the dorsum; no cnidosacs. Doto, British. Gellina. Heromorpha.
Fam. 7.—Fionidae. Dorsal papillae with a membranous expansion; male and female apertures at some distance from each other; pelagic. Fiona.
Fam. 8.—Pleurophyllidae. Anterior tentacles in the form of a digging shield; mantle without appendages, but respiratory papillae beneath the mantle-border. Pleurophyllidia.
Fam. 9.—Dermatobranchidae. Like the last, but wholly without branchiae. Dermatobranchus.
Tribe 4.—Elysiomorpha. Liver ramifies in integuments and extends into dorsal papillae, but there are no cnidosacs. Genital duct always triaulic, and male and female apertures distant from each other. No mandibles, and radula uniserial. Never more than one pair of tentacles, and these are absent in Alderia and some species of Limapontia.
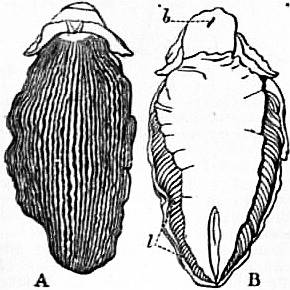 |
| Fig. 55.—Dorsal and Ventral View of Pleurophyllidia lineata (Otto), one of the Eolidomorph Nudibranchs. (After Keferstein.) |
b, The mouth. l, The lamelliform sub-pallial gills, which (as in Patella) replace the typical Molluscan ctenidium. |
Fam. 1.—Hermaeidae. Foot narrow; dorsal papillae linear or fusiform, in several series. Hermaea, British. Stiliger. Alderia, British.
Fam. 2.—Phyllobranchidae. Foot broad; dorsal papillae flattened and foliaceous. Phyllobranchus. Cyerce.
Fam. 3.—Plakobranchidae. Body depressed, without dorsal papillae, but with two very large lateral expansions, with dorsal plications. Plakobranchus.
Fam. 4.—Elysiidae. Body elongated, with lateral expansions; tentacles large; foot narrow. Elysia, British. Tridachia.
Fam. 5.—Limapontiidae. No lateral expansions, and no dorsal papillae; body planariform; anus dorsal, median and posterior. Limapontia, British. Actaeonia, British. Cenia.
Order 2 (of the Euthyneura).—Pulmonata. Euthyneurous Gastropoda, probably derived from ancestral forms similar to the Tectibranchiate Opisthobranchia by adaptation to a terrestrial life. The ctenidium is atrophied, and the edge of the mantle-skirt is fused to the dorsal integument by concrescence, except at one point which forms the aperture of the mantle-chamber, thus converted into a nearly closed sac. Air is admitted to this sac for respiratory and hydrostatic purposes, and it thus becomes a lung. An operculum is present only in Amphibola; a contrast being thus afforded with the operculate pulmonate Streptoneura (Cyclostoma, &c.), which differ in other essential features of structure from the Pulmonata. The Pulmonata are, like the other Euthyneura, hermaphrodite, with elaborately developed copulatory organs and accessory glands. Like other Euthyneura, they have very numerous small denticles on the lingual ribbon. In aquatic Pulmonata the osphradium is retained.
In some Pulmonata (snails) the foot is extended at right angles to the visceral hump, which rises from it in the form of a coil as in Streptoneura; in others the visceral hump is not elevated, but is extended with the foot, and the shell is small or absent (slugs).
 |
| Fig. 56.—A Series of Stylommatophorous Pulmonata, showing transitional forms between snail and slug. |
A, Helix pomatia. (From Keferstein.) B, Helicophanta brevipes. (From Keferstein, after Pfeiffer.) C, Testacella haliotidea. (From Keferstein.) D, Arion ater, the great black slug. (From Keferstein.) a, Shell in A, B, C, shell-sac (closed) in D; b, orifice leading into the sub-pallial chamber (lung). |
 |
| Fig. 57.—Ancylus fluviatilis, a patelliform aquatic Pulmonate. |
Pulmonata are widely distinguished from a small number of Streptoneura at one time associated with them on account of their mantle-chamber being converted, as in Pulmonata, into a lung, and the ctenidium or branchial plume aborted. The terrestrial Streptoneura (represented in England by the common genus Cyclostoma) have a twisted visceral nerve-loop, an operculum on the foot, a complex rhipidoglossate or taenio-glossate radula, and are of distinct sexes. The Pulmonata have a straight visceral nerve-loop, usually no operculum even in the embryo, and a multidenticulate radula, the teeth being equi-formal; and they are hermaphrodite. Some Pulmonata (Limnaea, &c.) live in fresh waters although breathing air. The remarkable discovery has been made that in deep lakes such Limnaei do not breathe air, but admit water to the lung-sac and live at the bottom. The lung-sac serves undoubtedly as a hydrostatic apparatus in the aquatic Pulmonata, as well as assisting respiration.
The same general range of body-form is shown in Pulmonata as in the Heteropoda and in the Opisthobranchia; at one extreme we have snails with coiled visceral hump, at the other cylindrical or flattened slugs (see fig. 56). Limpet-like forms are also found (fig. 57, Ancylus). The foot is always simple, with its flat crawling surface extending from end to end, but in the embryo Limnaea it shows a bilobed character, which leads on to the condition characteristic of Pteropoda.
The adaptation of the Pulmonata to terrestrial life has entailed little modification of the internal organization. In one genus (Planorbis) the plasma of the blood is coloured red by haemoglobin, this being the only instance of the presence of this body in the blood of Glossophorous Mollusca, though it occurs in corpuscles in the blood of the bivalves Arca and Solen (Lankester).
 |
| Fig. 58.—Hermaphrodite Reproductive Apparatus of the Garden Snail (Helix hortensis). |
τ, Ovo-testis. ve, Hermaphrodite duct. Ed, Albuminiparous gland. u, Uterine dilatation of the hermaphrodite duct. d, Digitate accessory glands on the female duct. ps, Calciferous gland or dart-sac on the female duct. Rf, Spermatheca or receptacle of the sperm in copulation, opening into the female duct. vd, Male duct (vas deferens). p, Penis. fl, Flagellum. |
The generative apparatus of the snail (Helix) may serve as an example of the hermaphrodite apparatus common to the Pulmonata and Opisthobranchia (fig. 58). From the ovo-testis, which lies near the apex of the visceral coil, a common hermaphrodite duct ve proceeds, which receives the duct of the compact white albuminiparous gland, Ed, and then becomes much enlarged, the additional width being due to the development of glandular folds, which are regarded as forming a uterus u. Where these folds cease the common duct splits into two portions, a male and a female. The male duct vd becomes fleshy and muscular near its termination at the genital pore, forming the penis p. Attached to it is a diverticulum fl, in which the spermatozoa which have descended from the ovo-testis are stored and modelled into sperm ropes or spermatophores. The female portion of the duct is more complex. Soon after quitting the uterus it is joined by a long duct leading from a glandular sac, the spermatheca (Rf). In this duct and sac the spermatophores received in copulation from another snail are lodged. In Helix hortensis the spermatheca is simple. In other species of Helix a second duct (as large in Helix aspersa as the chief one) is given off from the spermathecal duct, and in the natural state is closely adherent to the wall of the uterus. This second duct has normally no spermathecal gland at its termination, which is simple and blunt. But in rare cases in Helix aspersa a second spermatheca is found at the end of this second duct. Tracing the widening female duct onwards we now come to the openings of the digitate accessory glands d, d, which probably assist in the formation of the egg-capsule. Close to them is the remarkable dart-sac ps, a thick-walled sac, in the lumen of which a crystalline four-fluted rod or dart consisting of carbonate of lime is found. It is supposed to act in some way as a stimulant in copulation, but possibly has to do with the calcareous covering of the egg-capsule. Other Pulmonata exhibit variations of secondary importance in the details of this hermaphrodite apparatus.
The nervous system of Helix is not favourable as an example on account of the fusion of the ganglia to form an almost uniform ring of nervous matter around the oesophagus. The pond-snail (Limnaeus) furnishes, on the other hand, a very beautiful case of distinct ganglia and connecting cords (fig. 59). The demonstration which it affords of the extreme shortening of the Euthyneurous visceral nerve-loop is most instructive and valuable for comparison with and explanation of the condition of the nervous centres in Cephalopoda, as also of some Opisthobranchia. The figure (fig. 59) is sufficiently described in the letterpress attached to it; the pair of buccal ganglia joined by the connectives to the cerebrals are, as in most of our figures, omitted. Here we need only further draw attention to the osphradium, discovered by Lacaze-Duthiers, and shown by Spengel to agree in its innervation with that organ in all other Gastropoda. On account of the shortness of the visceral loop and the proximity of the right visceral ganglion to the oesophageal nerve-ring, the nerve to the osphradium and olfactory ganglion is very long. The position of the osphradium corresponds more or less closely with that of the vanished right ctenidium, with which it is normally associated. In Helix and Limax the osphradium has not been described, and possibly its discovery might clear up the doubts which have been raised as to the nature of the mantle-chamber of those genera. In Planorbis, which is sinistral (as are a few other genera or exceptional varieties of various Anisopleurous Gastropods), instead of being dextral, the osphradium is on the left side, and receives its nerve from the left visceral ganglion, the whole series of unilateral organs being reversed. This is, as might be expected, what is found to be the case in all “reversed” Gastropods.
The shell of the Pulmonata, though always light and delicate, is in many cases a well-developed spiral “house” into which the creature can withdraw itself; and, although the foot possesses no operculum, yet in Helix the aperture of the shell is closed in the winter by a complete lid, the “hybernaculum” more or less calcareous in nature, which is secreted by the foot. In Clausilia a peculiar modification of this lid exists permanently in the adult, attached by an elastic stalk to the mouth of the shell, and known as the “clausilium.” In Limnaeus the permanent shell is preceded in the embryo by a well-marked shell-gland or primitive shell-sac (fig. 60), at one time supposed to be the developing anus, but shown by Lankester to be identical with the “shell-gland” discovered by him in other Mollusca (Pisidium, Pleurobranchidium, Neritina, &c.). As in other Gastropoda Anisopleura, this shell-sac may abnormally develop a plug of chitinous matter, but normally it flattens out and disappears, whilst the cap-like rudiment of the permanent shell is shed out from the dome-like surface of the visceral hump, in the centre of which the shell-sac existed for a brief period.
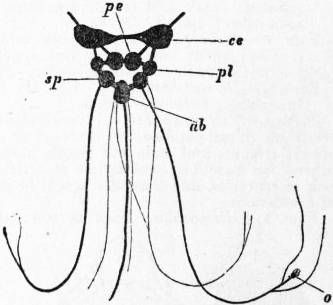 |
| Fig. 59.—Nervous System of the Pond-Snail, Limnaeus stagnalis, as a type of the short-looped euthyneurous condition. The short visceral “loop” with its three ganglia is lightly-shaded. |
ce, Cerebral ganglion. pe, Pedal ganglion. pl, Pleural ganglion. ab, Abdominal ganglion. sp, Visceral ganglion of the left side; opposite to it is the visceral ganglion of the right side, which gives off the long nerve to the olfactory ganglion and osphradium o. |
| In Planorbis and in Auricula (Pulmonata, allied to Limnaeus) the olfactory organ is on the left side and receives its nerve from the left visceral ganglion. (After Spengel.) |
In Clausilia, according to the observations of C. Gegenbaur, the primitive shell-sac does not flatten out and disappear, but takes the form of a flattened closed sac. Within this closed sac a plate of calcareous matter is developed, and after a time the upper wall of the sac disappears, and the calcareous plate continues to grow as the nucleus of the permanent shell. In the slug Testacella (fig. 56, C) the shell-plate never attains a large size, though naked. In other slugs, namely, Limax and Arion, the shell-sac remains permanently closed over the shell-plate, which in the latter genus consists of a granular mass of carbonate of lime. The permanence of the primitive shell-sac in these slugs is a point of considerable interest. It is clear enough that the sac is of a different origin from that of Aplysia (described in the section treating of Opisthobranchia), being primitive instead of secondary. It seems probable that it is identical with one of the open sacs in which each shell-plate of a Chiton is formed, and the series of plate-like imbrications which are placed behind the single shell-sac on the dorsum of the curious slug, Plectrophorus, suggest the possibility of the formation of a series of shell-sacs on the back of that animal similar to those which we find in Chiton. Whether the closed primitive shell-sac of the slugs (and with it the transient embryonic shell-gland of all other Mollusca) is precisely the same thing as the closed sac in which the calcareous pen or shell of the Cephalopod Sepia and its allies is formed, is a further question which we shall consider when dealing with the Cephalopoda. It is important here to note that Clausilia furnishes us with an exceptional instance of the continuity of the shell or secreted product of the primitive shell-sac with the adult shell. In most other Mollusca (Anisopleurous Gastropods, Pteropods and Conchifera) there is a want of such continuity; the primitive shell-sac contributes no factor to the permanent shell, or only a very minute knob-like particle (Neritina and Paludina). It flattens out and disappears before the work of forming the permanent shell commences. And just as there is a break at this stage, so (as observed by A. Krohn in Marsenia = Echinospira) there may be a break at a later stage, the nautiloid shell formed on the larva being cast, and a new shell of a different form being formed afresh on the surface of the visceral hump. It is, then, in this sense that we may speak of primary, secondary and tertiary shells in Mollusca recognizing the fact that they may be merely phases fused by continuity of growth so as to form but one shell, or that in other cases they may be presented to us as separate individual things, in virtue of the non-development of the later phases, or in virtue of sudden changes in the activity of the mantle-surface causing the shedding or disappearance of one phase of shell-formation before a later one is entered upon.
The development of the aquatic Pulmonata from the egg offers considerable facilities for study, and that of Limnaeus has been elucidated by E.R. Lankester, whilst H. Rabl has with remarkable skill applied the method of sections to the study of the minute embryos of Planorbis. The chief features in the development of Limnaeus are exhibited in fig. 60. There is not a very large amount of food-material present in the egg of this snail, and accordingly the cells resulting from division are not so unequal as in many other cases. The four cells first formed are of equal size, and then four smaller cells are formed by division of these four so as to lie at one end of the first four (the pole corresponding to that at which the “directive corpuscles” are extruded and remain). The smaller cells now divide and spread over the four larger cells; at the same time a space—the cleavage cavity or blastocoel—forms in the centre of the mulberry-like mass. Then the large cells recommence the process of division and sink into the hollow of the sphere, leaving an elongated groove, the blastopore, on the surface. The invaginated cells (derived from the division of the four big cells) form the endoderm or arch-enteron; the outer cells are the ectoderm. The blastopore now closes along the middle part of its course, which coincides in position with the future “foot.” One end of the blastopore becomes nearly closed, and an ingrowth of ectoderm takes place around it to form the stomodaeum or fore-gut and mouth. The other extreme end closes, but the invaginated endoderm cells remain in continuity with this extremity of the blastopore, and form the “rectal peduncle” or “pedicle of invagination” of Lankester, although the endoderm cells retain no contact with the middle region of the now closed-up blastopore. The anal opening forms at a late period by a very short ingrowth or proctodaeum coinciding with the blind termination of the rectal peduncle (fig. 60, pi).
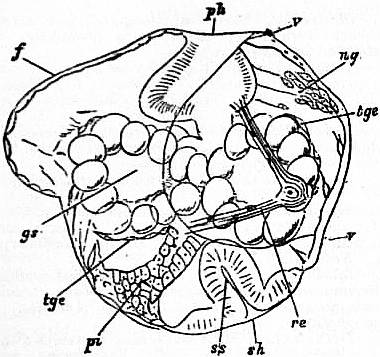 |
|
| Fig. 60.—Embryo of Limnaeus stagnalis, at a stage when the Trochosphere is developing foot and shell-gland and becoming a Veliger, seen as a transparent object under slight pressure. (Lankester.) | |
ph, Pharynx (stomodaeal invagination). v, v, The ciliated band marking out the velum. ng, Cerebral nerve-ganglion. re, Stiebel’s canal (left side), probably an evanescent embryonic nephridium. sh, The primitive shell-sac or shell-gland. |
pi, The rectal peduncle or pedicle of invagination; its attachment to the ectoderm is coincident with the hindmost extremity of the elongated blastopore of fig. 3, C. tge, Mesoblastic (skeleto-trophic and muscular) cells investing gs, the bilobed arch-enteron or lateral vesicles of invaginated endoderm, which will develop into liver. f, The foot. |
The body-cavity and the muscular, fibrous and vascular tissues are traced partly to two symmetrically disposed “mesoblasts,” which bud off from the invaginated arch-enteron, partly to cells derived from the ectoderm, which at a very early stage is connected by long processes with the invaginated endoderm. The external form of the embryo goes through the same changes as in other Gastropods, and is not, as was held previously to Lankester’s observations, exceptional. When the middle and hinder regions of the blastopore are closing in, an equatorial ridge of ciliated cells is formed, converting the embryo into a typical trochosphere.
The foot now protrudes below the mouth, and the post-oral hemisphere of the trochosphere grows more rapidly then the anterior or velar area. The young foot shows a bilobed form. Within the velar area the eyes and the cephalic tentacles commence to rise up, and on the surface of the post-oral region is formed a cap-like shell and an encircling ridge, which gradually increases in prominence and becomes the freely depending mantle-skirt. The outline of the velar area becomes strongly emarginated and can be traced through the more mature embryos to the cephalic lobes or labial processes of the adult Limnaeus (fig. 61).
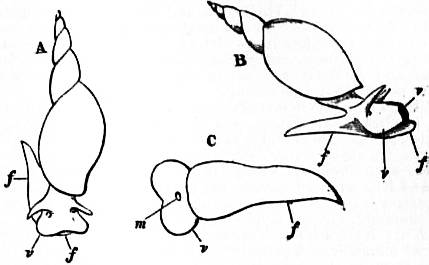 |
| Fig. 61.—A, B, C. Three views of Limnaeus stagnalis, in order to show the persistence of the larval velar area v, as the circum-oral lobes of the adult. m, Mouth; f, foot; v, velar area, the margin v corresponding with the ciliated band which demarcates the velar area or velum of the embryo Gastropod (see fig. 4, D, E, F, H, I, v). (Original.) |
The increase of the visceral dome, its spiral twisting, and the gradual closure of the space overhung by the mantle-skirt so as to convert it into a lung-sac with a small contractile aperture, belong to stages in the development later than any represented in our figures.
We may now revert briefly to the internal organization at a period when the trochosphere is beginning to show a prominent foot growing out from the area where the mid-region of the elongated blastopore was situated, and having therefore at one end of it the mouth and at the other the anus. Fig. 60 represents such an embryo under slight compression as seen by transmitted light. The ciliated band of the left side of the velar area is indicated by a line extending from v to v; the foot f is seen between the pharynx ph and the pedicle of invagination pi. The mass of the arch-enteron or invaginated endodermal sac has taken on a bilobed form, and its cells are swollen (gs and tge). This bilobed sac becomes entirely the liver in the adult; the intestine and stomach are formed from the pedicle of invagination, whilst the pharynx, oesophagus and crop form from the stomodaeal invagination ph. To the right (in the figure) of the rectal peduncle is seen the deeply invaginated shell-gland ss, with a secretion sh protruding from it. The shell-gland is destined in Limnaeus to become very rapidly stretched out, and to disappear. Farther up, within the velar area, the rudiments of the cerebral nerve-ganglion ng are seen separating from the ectoderm. A remarkable cord of cells having a position just below the integument occurs on each side of the head. In the figure the cord of the left side is seen, marked re. This paired organ consists of a string of cells which are perforated by a duct opening to the exterior and ending internally in a flame-cell. Such cannulated cells are characteristic of the nephridia of many worms, and the organs thus formed in the embryo Limnaeus are embryonic nephridia. The most important fact about them is that they disappear, and are in no way connected with the typical nephridium of the adult. In reference to their first observer they were formerly called “Stiebel’s canals.” Other Pulmonata possess, when embryos, Stiebel’s canals in a more fully developed state, for instance, the common slug Limax. Here too they disappear during embryonic life. Similar larval nephridia occur in other Gastropoda. In the marine Streptoneura they are ectodermic projections which ultimately fall off; in the Opisthobranchs they are closed pouches; in Paludina and Bithynia they are canals as in Pulmonata.
 |
| Fig. 62.—Oncidium tonganum, a littoral Pulmonate, found on the shores of the Indian and Pacific Oceans (Mauritius, Japan). |
Marine Pulmonata.—Whilst the Pulmonata are essentially a terrestrial and fresh-water group, there is one genus of slug-like Pulmonates which frequent the sea-coast (Oncidium, fig. 62). Karl Semper has shown that these slugs have, in addition to the usual pair of cephalic eyes, a number of eyes developed upon the dorsal integument. These dorsal eyes are very perfect in elaboration, possessing lens, retinal nerve-end cells, retinal pigment and optic nerve. Curiously enough, however, they differ from the cephalic Molluscan eye in the fact that, as in the vertebrate eye, the filaments of the optic nerve penetrate the retina, and are connected with the surfaces of the nerve-end cells nearer the lens instead of with the opposite end. The significance of this arrangement is not known, but it is important to note, as shown by V. Henson, S.J. Hickson and others, that in the bivalves Pecten and Spondylus, which also have eyes upon the mantle quite distinct from typical cephalic eyes, there is the same relationship as in Oncidiidae of the optic nerve to the retinal cells. In both Oncidiidae and Pecten the pallial eyes have probably been developed by the modification of tentacles, such as coexist in an unmodified form with the eyes. The Oncidiidae are, according to K. Semper, pursued as food by the leaping fish Periophthalmus, and the dorsal eyes are of especial value to them in aiding them to escape from this enemy.
Sub-order 1.—Basommatophora. Pulmonata with an external shell. The head bears a single pair of contractile but not invaginable tentacles, at the base of which are the eyes. Penis at some distance from the female aperture, except in Amphibola and Siphonaria. All have an osphradium, except the Auriculidae, which are terrestrial, and it is situated outside the pallial cavity in those forms in which water is not admitted into the lung. There is a veliger stage in development, but the velum is reduced.
Fam. 1.—Auriculidae. Terrestrial and usually littoral; genital duct monaulic, the penis being connected with the aperture by an open or closed groove; shell with a prominent spire, the internal partitions often absorbed and the aperture denticulated. Auricula. Cassidula. Alexia. Melampus. Carychium, terrestrial, British. Scarabus. Leuconia, British. Blauneria. Pedipes.
Fam. 2.—Otinidae. Shell with short spire, and wide oval aperture; tentacles short. Otina, British. Camptonyx, terrestrial.
Fam. 3.—Amphibolidae. Shell spirally coiled; head broad, without prominent tentacles; foot short, operculated; marine. Amphibola.
Fam. 4.—Siphonariidae. Visceral mass and shell conical; tentacles atrophied; head expanded; genital apertures contiguous; marine animals, with an aquatic pallial cavity containing secondary branchial laminae. Siphonaria.
Fam. 5.—Gadiniidae. Visceral mass and shell conical; head flattened; pallial cavity aquatic, but without a branchia; genital apertures separated. Gadinia.
Fam. 6.—Chilinidae. Shell ovoid, with short spire, wide aperture and folded columella; inferior pallial lobe thick; visceral commissure still twisted. Chilina.
Fam. 7.—Limnaeidae. Shell thin, dextral, with prominent spire and oval aperture; no inferior pallial lobe. Limnaea, British. Amphipeplea, British.
Fam. 8.—Pompholygidae. Shell dextral, hyperstrophic, animal sinistral. Pompholyx. Choanomphalus.
Fam. 9.—Planorbidae. Visceral mass and shell sinistral; inferior pallial lobe very prominent, and transformed into a branchia. Planorbis, British. Bulinus. Miratesta.
Fam. 10.—Ancylidae. Shell conical, not spiral; inferior pallial lobe transformed into a branchia. Ancylus, British. Latia. Grundlachia.
Fam. 11.—Physidae. Visceral mass and shell sinistrally coiled; shell thin, with narrow aperture; no inferior pallial lobe. Physa, British. Aplexa, British.
Sub-order 2.—Stylommatophora. Pulmonata with two pairs of tentacles, except Janellidae and Vertigo; these tentacles are invaginable, and the eyes are borne on the summits of the posterior pair. Male and female genital apertures open into a common vestibule, except in Vaginulidae and Oncidiidae. Except in Oncidium, there is no longer a veliger stage in development.
Tribe 1.—Holognatha. Jaw simple, without a superior appendage.
Fam. 1.—Selenitidae. Radula with elongated and pointed teeth, like those of the Agnatha; a jaw present. Plutonia. Trigonochlamys.
Fam. 2.—Zonitidae. Shell external, smooth, heliciform or flattened; radula with pointed marginal teeth. Zonites, British. Ariophanta. Orpiella. Vitrina. Helicarion.
Fam. 3.—Limacidae. Shell internal. Limax, British. Parmacella. Urocyclus. Parmarion. Amalia. Agriolimax. Mesolimax. Monochroma. Paralimax. Metalimax.
Fam. 4.—Philomycidae. No shell; mantle covers the whole surface of the body; radula with squarish teeth. Philomycus.
Fam. 5.—Ostracolethidae. Shell largely chitinous, not spiral, its calcareous apex projecting through a small hole in the mantle. Ostracolethe.
Fam. 6.—Arionidae. Shell internal, or absent; mantle restricted to the anterior and middle part of the body; radula with squarish teeth. Arion, British. Geomalacus. Ariolimax. Anadenus.
Fam. 7.—Helicidae. Shell with medium spire, external or partly covered by the mantle; genital aperture below the right posterior tentacle; genital apparatus generally provided with a dart-sac and multifid vesicles. Helix, British. Bulimus. Hemphillia. Berendtia. Cochlostyla. Rhodea.
Fam. 8.—Endodontidae. Shell external, spiral, generally ornamented with ribs; borders of aperture thin and not reflected; radula with square teeth; genital ducts without accessory organs. Endodonta. Punctum. Sphyradium. Laoma. Pyramidula.
Fam. 9.—Orthalicidae. Shell external, ovoid, the last whorl swollen, aperture oval with a simple border; radular teeth in oblique rows. Orthalicus.
Fam. 10.—Bulimulidae. Jaw formed of folds imbricated externally and meeting at an acute angle near the base. Bulimulus. Peltella. Amphibulimus.
Fam. 11.—Cylindrellidae. Shell turriculated, with numerous whorls, the last more or less detached. Cylindrella.
Fam. 12.—Pupidae. Shell external, with elongated spire and numerous whorls, aperture generally narrow; male genital duct without multifid vesicles. Pupa, British. Eucalodium. Vertigo, British. Buliminus, British. Clausilia, British. Balea. Zospeum. Megaspira. Strophia. Anostoma.
Fam. 13.—Stenogyridae. Shell elongated, with a more or less obtuse summit; aperture with a simple border. Achatina. Stenogyra. Ferussacia, British. Cionella. Caecilianella. Azeca. Opeas.
Fam. 14.—Helicteridae. Shell bulimoid, dextral or sinistral; radular teeth, expanded at their extremities and multicuspidate. Helicter. Tornatellina.
Tribe 2.—Agnatha. No jaws; teeth narrow and pointed; carnivorous.
Fam. 1.—Oleacinidae. Shell oval, elongated, with narrow aperture; neck very long; labial palps prominent. Oleacina (Glandina). Streptostyla.
Fam. 2.—Testacellidae. Shell globular or auriform, external or partly covered by the mantle. Streptaxis. Gibbulina. Aerope. Rhytida. Daudebardia. Testacella. Chlamydophorus. Schizoglossa.
Fam. 3.—Rathouisiidae. No shell, a carinated mantle covering the whole body; male and female apertures distant, the female near the anus. Rathouisia. Atopos.
Tribe 3.—Elasmognatha. Jaw with a well-developed dorsal appendage.
Fam. 1.—Succineidae. Anterior tentacles much reduced; male and female apertures contiguous but distinct; shell thin, spiral, with short spire. Succinea, British. Homalonyx. Hyalimax. Neohyalimax.
Fam. 2.—Janellidae. Limaciform, with internal rounded shell; mantle very small and triangular; pulmonary chamber with tracheae; no anterior tentacles. Janella. Aneitella. Aneitea. Triboniophorus.
Tribe 4.—Ditremata. Male and female apertures distant.
Fam. 1.—Vaginulidae. No shell; limaciform; terrestrial; female aperture on right side in middle of body; anus posterior. Vaginula.
Fam. 2.—Oncidiidae. No shell; limaciform; littoral; female aperture posterior, near anus; a reduced pulmonary cavity with a distinct aperture. Oncidium. Oncidiella, British. Peronia.
Authorities.—L. Boutan, “La Cause principale de l’asymétrie des mollusques gastéropodes,” Arch. de zool. expér. (3), vii. (1899); A. Lang, “Versuch einer Erklärung der Asymmetrie der Gastropoder,” Vierteljahrsschr. naturforsch. Gesellschaft, Zürich, 36 (1892); A. Robert, “Recherches sur le développement des Troques,” Arch. de zool. expér. (3), x. (1903); P. Pelseneer, “Report on the Pteropoda,” Zool. “Challenger” Expedit. pts. lviii., lxv., lxvi. (1887, 1888); P. Pelseneer, “Protobranches aériens et Pulmonés branchifères,” Arch. de biol. xiv. (1895); W.A. Herdman, “On the Structure and Functions of the Cerata or Dorsal Papillae in some Nudibranchiate Mollusca,” Quart. Journ. Mic. Sci. (1892); J.T. Cunningham, “On the Structure and Relations of the Kidney in Aplysia,” Mitt. Zool. Stat. Neapel, iv. (1883); Böhmig, “Zur feineren Anatomie von Rhodope veranyi, Kölliker,” Zeitschr. f. wiss. Zool. vol. lvi. (1893).
Treatises.—S.P. Woodward, Manual of the Mollusca (2nd ed., with appendix, London, 1869); E. Forbes and S. Hanley, History of British Mollusca (4 vols., London, 1853); Alder and Hancock, Monograph of British Nudibranchiate Mollusca (London, Roy. Society, 1845); P. Pelseneer, Mollusca. Treatise on Zool., edited by E. Ray Lankester, pt. v. (1906); E. Ray Lankester, “Mollusca,” in 9th ed. of this Encyclopaedia, to which this article is much indebted.
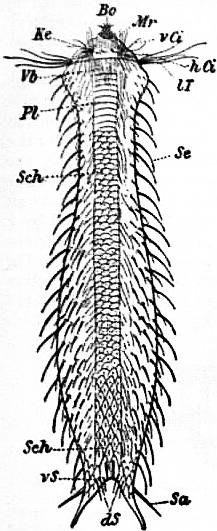 |
| From Zeitschrift für Wissenschaft Zoologie, vol. xlix. p. 209, by permission of Wilhelm Engelmann. |
| Chaetonotus maximus, Ehrb., ventral side. (After Zelinka.) |
Bo, Bristles surrounding the mouth. ds, Dorsal bristles. hCi, Posterior lateral cilia. Ke, Cuticular dome. Mr, Oral cavity. lT, Lateral sensory hairs. Pl, Cuticular plates. Sa, Dorsal bristle of the basal part. Sch, Plates. Se, Lateral bristles. Vb, Point of union of ciliated tract. vCi, Anterior group of cilia. vS, Ventral bristles of the basal part. |