Galls. In animals galls occur mostly on or under the skin of living mammals and birds, and are produced by Acaridea, and by dipterous insects of the genus Oestrus. Signor Moriggia1 has described and figured a horny excrescence, nearly 8 in. in length, from the back of the human hand, which was caused by Acarus domesticus. What are commonly known as galls are vegetable excrescences, and, according to the definition of Lacaze-Duthiers, comprise “all abnormal vegetable productions developed on plants by the action of animals, more particularly by insects, whatever may be their form, bulk or situation.” For the larvae of their makers the galls provide shelter and sustenance. The exciting cause of the hypertrophy, in the case of the typical galls, appears to be a minute quantity of some irritating fluid, or virus, secreted by the female insect, and deposited with her egg in the puncture made by her ovipositor in the cortical or foliaceous parts of plants. This virus causes the rapid enlargement and subdivision of the cells affected by it, so as to form the tissues of the gall. Oval or larval irritation also, without doubt, plays an important part in the formation of many galls. Though, as Lacaze-Duthiers remarks, a certain relation is necessary between the “stimulus” and the “supporter of the stimulus,” as evidenced by the limitation in the majority of cases of each species of gall-insect to some one vegetable structure, still it must be the quality of the irritant of the tissues, rather than the specific peculiarities or the part of the plant affected, that principally determines the nature of the gall. Thus the characteristics of the currant-gall of Spathegaster baccarum, L., which occurs alike on the leaves and on the flower-stalks of the oak, are obviously due to the act of oviposition, and not to the functions of the parts producing it; the bright red galls of the saw-fly Nematus gallicola are found on four different species of willow, Salix fragilis, S. alba, S. caprea and S. cinerea;2 and the galls of a Cynipid, Biorhiza aptera, usually developed on the rootlets of the oak, have been procured also from the deodar.3 Often the gall bears no visible resemblance to the structures out of which it is developed; commonly, however, outside the larval chamber, or gall proper, and giving to the gall its distinctive form, are to be detected certain more or less modified special organs of the plant. The gall of Cecidomyia strobilina, formed from willow-buds, is mainly a rosette of leaves the stalks of which have had their growth arrested. The small, smooth, seed-shaped gall of the American Cynips seminator, Harris, according to W.F. Bassett,4 is the petiole, and its terminal tuft of woolly hairs the enormously developed pubescence of the young oak-leaf. The moss-like covering of the “bedeguars” of the wild rose, the galls of a Cynipid, Rhodites rosae, represents leaves which have been developed with scarcely any parenchyma between their fibro-vascular bundles; and the “artichoke-galls” or “oak-strobile,” produced by Aphilothrix gemmae, L., which insect arrests the development of the acorn, consists of a cupule to which more or less modified leaf-scales are attached, with a peduncular, oviform, inner gall.5 E. Newman held the view that many oak-galls are pseudobalani or false acorns: “to produce an acorn has been the intention of the oak, but the gall-fly has frustrated the attempt.” Their formation from buds which normally would have yielded leaves and shoots is explained by Parfitt as the outcome of an effort at fructification induced by oviposition, such as has been found to result in several plants from injury by insect-agency or otherwise.6 Galls vary remarkably in size and shape according to the species of their makers. The polythalamous gall of Aphilothrix radicis, found on the roots of old oak-trees, may attain the size of a man’s fist; the galls of another Cynipid, Andricus occultus, Tschek,7 which occurs on the male flowers of Quercus sessiliflora, is 2 millimetres, or barely a line, in length. Many galls are brightly coloured, as, for instance, the oak-leaf hairy galls of Spathegaster tricolor, which are of a crimson hue, more or less diffused according to exposure to light. The variety of forms of galls is very great. Some are like urns or cups, others lenticular. The “knoppern” galls of Cynips polycera, Gir., are cones having the broad, slightly convex upper surface surrounded with a toothed ridge. Of the Ceylonese galls, “some are as symmetrical as a composite flower when in bud, others smooth and spherical like a berry; some protected by long spines, others clothed with yellow wool formed of long cellular hairs, others with regularly tufted hairs.”8 The characters of galls are constant, and as a rule exceedingly diagnostic, even when, as in the case of ten different gall-gnats of an American willow, Salix humilis, it is difficult or impossible to tell the full-grown insects that produce them from one another. In degree of complexity of internal structure galls differ considerably. Some are monothalamous, and contain but one larva of the gall-maker, whilst others are many-celled and numerously inhabited. The largest class are the unilocular, or simple, external galls, divided by Lacaze-Duthiers into those with and those without a superficial protective layer or rind, and composed of hard, or spongy, or cellular tissue. In a common gall-nut that authority distinguished seven constituent portions: an epidermis; a subdermic cellular tissue; a spongy and a hard layer, composing the parenchyma proper; vessels which, without forming a complete investment, underlie the parenchyma; a hard protective layer; and lastly, within that, an alimentary central mass inhabited by the growing larva.9
Galls are formed by insects of several orders. Among the Hymenoptera are the gall-wasps (Cynips and its allies), which infect the various species of oak. They are small insects, having straight antennae, and a compressed, usually very short abdomen with the second or second and third segments greatly developed, and the rest imbricated, and concealing the partially coiled ovipositor. The transformations from the larval state are completed within the gall, out of which the imago, or perfect insect, tunnels its way,—usually in autumn, though sometimes, as has been observed of some individuals of Cynips Kollari, after hibernation.
Among the commoner of the galls of the Cynipidae are the “oak-apple” or “oak-sponge” of Andricus terminalis, Fab.; the “currant” or “berry galls” of Spathegaster baccarum, L., above mentioned; and the “oak-spangles” of Neuroterus lenticularis,10 Oliv., generally reputed to be fungoid growths, until the discovery of their true nature by Frederick Smith,11 and the succulent “cherry-galls” of Dryophanta scutellaris, Oliv. The “marble” or “Devonshire woody galls” of oak-buds, which often destroy the leading shoots of young trees, are produced by Cynips Kollari,12 already alluded to. They were first introduced into Devonshire about the year 1847, had become common near Birmingham by 1866, and two or three years later were observed in several parts of Scotland.13 They contain about 17% of tannin.14 On account of their regular form they have been used, threaded on wire, for making ornamental baskets. The large purplish Mecca or Bussorah galls,15 produced on a species of oak by Cynips insana, Westw., have been regarded by many writers as the Dead Sea fruit, mad-apples (mala insana), or apples of Sodom (poma sodomitica), alluded to by Josephus and others, which, however, are stated by E. Robinson (Bibl. Researches in Palestine, vol. i. pp. 522-524, 3rd ed., 1867) to be the singular fruit called by the Arabs ’Ösher, produced by the Asclepias gigantea or procera of botanists. What in California are known as “flea seeds” are oak-galls made by a species of Cynips; in August they become detached from the leaves that bear them, and are caused to jump by the spasmodic movements of the grub within the thin-walled gall-cavity.16
Common gall-nuts, nut-galls, or oak-galls, the Aleppo, Turkey, or Levant galls of commerce (Ger. Galläpfel, levantische Gallen; Fr. noix de Galle), are produced on Quercus infectoria, a variety of Q. Lusitanica, Webb, by Cynips (Diplolepis, Latr.) tinctoria, L., or C. gallae tinctoriae Oliv. Aleppo galls (gallae halepenses) are brittle, hard, spherical bodies, 2⁄5-4⁄5 in. in diameter, ridged and warty on the upper half, and light brown to dark greyish-yellow within. What are termed “blue,” “black,” or “green” galls contain the insect; the inferior “white” galls, which are lighter coloured, and not so compact, heavy or astringent, are gathered after its escape (see fig. 1.). Less valued are the galls of Tripoli (Taraplus or Tarabulus, whence the name “Tarablous galls”). The most esteemed Syrian galls, according to Pereira, are those of Mosul on the Tigris. Other varieties of nut-galls, besides the above-mentioned, are employed in Europe for various purposes. Commercial gall-nuts have yielded on analysis from 26 (H. Davy) to 77 (Buchner) % of tannin (see Vinen, loc. cit.), with gallic and ellagic acids, ligneous fibre, water, and minute quantities of proteids, chlorophyll, resin, free sugar and, in the cells around the inner shelly chamber, calcium oxalate. Oak-galls are mentioned by Theophrastus, Dioscorides (i. 146), and other ancient writers, including Pliny (Nat. Hist. xvi. 9, 10, xxiv. 5), according to whom they may be produced “in a single night.” Their insect origin appears to have been entirely unsuspected until within comparatively recent times, though Pliny, indeed, makes the observation that a kind of gnat is produced in certain excrescences on oak leaves. Bacon describes oak-apples as “an exudation of plants joined with putrefaction.” Pomet17 thought that gall-nuts were the fruit of the oak, and a similar opinion obtains among the modern Chinese, who apply to them the term Mu-shih-tsze, or “fruits for the foodless.”18 Hippocrates administered gall-nuts for their astringent properties, and Pliny (Nat. Hist. xxiv. 5) recommends them as a remedy in affections of the gums and uvula, ulcerations of the mouth and some dozen more complaints. In British pharmacy gall-nuts are used in the preparation of the two astringent ointments unguentum gallae and unguentum gallae cum opio, and of the tinctura gallae, and also as a source of tannin and of gallic acid (q.v.). They have from very early times been resorted to as a means of staining the hair of a dark colour, and they are the base of the tattooing dye of the Somali women.19
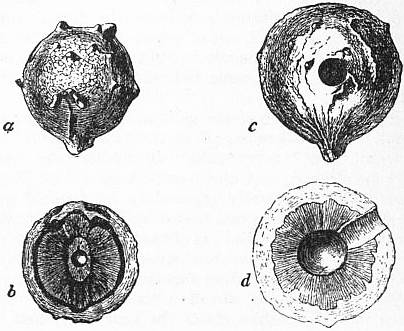 |
| Fig. 1.—a, Aleppo “blue” gall; b, ditto in section, showing central cavity for grub; c, Aleppo “white” gall, perforated by insect; d, the same in section (natural size). |
The gall-making Hymenoptera include, besides the Cynipidae proper, certain species of the genus Eurytoma (Isosoma, Walsh) and family Chalcididae, e.g. E. hordei, the “joint-worm” of the United States, which produces galls on the stalks of wheat;20 also various members of the family Tenthredinidae, or saw-flies. The larvae of the latter usually vacate their galls, to spin their cocoons in the earth, or, as in the case of Athalia abdominalis, Klg., of the clematis, may emerge from their shelter to feed for some days on the leaves of the gall-bearing plant.
The dipterous gall-formers include the gall-midges, or gall-gnats (Cecidomyidae), minute slender-bodied insects, with bodies usually covered with long hairs, and the wings folded over the back. Some of them build cocoons within their galls, others descend to the ground or become pupae. The true willow-galls are the work either of these or of saw-flies. Their galls are to be met with on a great variety of plants of widely distinct genera, e.g. the ash, maple, horn-beam, oak,21 grape-vine,22 alder, gooseberry, blackberry, pine, juniper, thistle, fennel, meadowsweet,23 common cabbage and cereals. In the northern United States, in May, “legions of these delicate minute flies fill the air at twilight, hovering over wheat-fields and shrubbery. A strong north-west wind, at such times, is of incalculable value to the farmer.”24 Other gall-making dipterous flies are members of the family Trypetidae, which disfigure the seed-heads of plants, and of the family Mycetophilidae, such as the species Sciara tilicola,25 Löw, the cause of the oblong or rounded green and red galls of the young shoots and leaves of the lime.
Galls are formed also by hemipterous and homopterous insects of the families Tingidae, Psyllidae, Coccidae and Aphidae. Coccus pinicorticis causes the growth of patches of white flocculent and downy matter on the smooth bark of young trees of the white pine in America.26 The galls of examples of the last family are common objects on lime-leaves, and on the petioles of the poplar. An American Aphid of the genus Pemphigus produces black, ragged, leathery and cut-shaped excrescences on the young branches of the hickory.
The Chinese galls of commerce (Woo-pei-tsze) are stated to be produced by Aphis Chinensis, Bell, on Rhus semialata, Murr. (R. Bucki-amela, Roxb.), an Anacardiaceous tree indigenous to N. India, China and Japan. They are hollow, brittle, irregularly pyriform, tuberculated or branched vesicles, with thin walls, covered externally with a grey down, and internally with a white chalk-like matter, and insect-remains (see fig. 2). The escape of the insect takes place on the spontaneous bursting of the walls of the vesicle, probably when, after viviparous (thelytokous) reproduction for several generations, male winged insects are developed. The galls are gathered before the frosts set in, and are exposed to steam to kill the insects.27
Chinese galls examined by Viedt28 yielded 72% of tannin, and less mucilage than Aleppo galls. Several other varieties of galls are produced by Aphides on species of Pistacia.
M.J. Lichtenstein has established the fact that from the egg of the Aphis of Pistachio galls, Anopleura lentisci, is hatched an apterous insect (the gall-founder), which gives birth to young Aphides (emigrants), and that these, having acquired wings, fly to the roots of certain grasses (Bromus sterilis and Hordeum vulgare), and by budding underground give rise to several generations of apterous insects, whence finally comes a winged brood (the pupifera). These last issuing from the ground fly to the Pistachio, and on it deposit their pupae. From the pupae, again, are developed sexual individuals, the females of which lay fecundated eggs productive of gall-founders, thus recommencing the biological cycle (see Compt. rend., Nov. 18, 1878, p. 782, quoted in Ann. and Mag. Nat. Hist., 1879, p. 174).
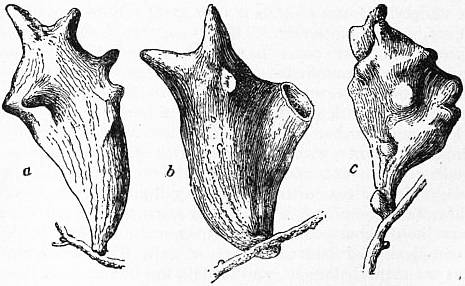 |
| Fig. 2.—a, Chinese gall (abt. ½ natural size); b, ditto broken, showing thin-walled cavity; c, Japanese gall (natural size). |
Of other insects which have been recognized as gall-makers there are, among the Coleoptera, certain Curculionids (gall-weevils), and species of the exotic Sagridae and Lamiadae and an American beetle, Saperda inornata (Cerambycidae), which forms the pseudo-galls of Salix longifolia and Populus angulata, or cottonwood. Among the Lepidoptera are gall-forming species belonging to the Tineidae, Aegeriidae, Tortricidae and Pterophoridae. The larva of a New Zealand moth, Morova subfasciata, Walk. (Cacoëcia gallicolens), of the family Drepanulidae, causes the stem of a creeping plant, on the pith of which it apparently subsists, to swell up into a fusiform gall.29
Mite-galls, or acarocecidia, are abnormal growths of the leaves of plants, produced by microscopic Acaridea of the genus Phytoptus (gall-mites), and consist of little tufts of hairs, or of thickened portions of the leaves, usually most hypertrophied on the upper surface, so that the lower is drawn up into the interior, producing a bursiform cavity. Mite-galls occur on the sycamore, pear, plum, ash, alder, vine, mulberry and many other plants; and formerly, e.g. the gall known as Erineum quercinum, on the leaves of Quercus Cerris, were taken for cryptogamic structures. The lime-leaf “nail-galls” of Phytoptus tiliae closely resemble the “trumpet-galls” formed on American vines by a species of Cecidomyia.30 Certain minute Nematoid worms, as Anguillula scandens, which infests the ears of wheat, also give rise to galls.
Besides the larva of the gall-maker, or the householder, galls usually contain inquilines or lodgers, the larvae of what are termed guest-flies or cuckoo-flies. Thus the galls of Cynips and its allies are inhabited by members of other cynipideous genera, as Synergus, Amblynotus and Synophrus; and the pine-cone-like gall of Salix strobiloides, as Walsh has shown,30 is made by a large species of Cecidomyia, which inhabits the heart of the mass, the numerous smaller cecidomyidous larvae in its outer part being mere inquilines. In many instances the lodgers are not of the same order of insects as the gall-makers. Some saw-flies, for example, are inquilinous in the galls of gall-gnats and some gall-gnats in the galls of saw-flies. Again, galls may afford harbour to insects which are not essentially gall-feeders, as in the case of the Curculio beetle Conotrachelius nenuphar, Hbst., of which one brood eats the fleshy part of the plum and peach, and another lives in the “black knot” of the plum-tree, regarded by Walsh as probably a true cecidomyidous gall. The same authority (loc. cit. p. 550) mentions a willow-gall which provides no less than sixteen insects with food and protection; these are preyed upon by about eight others, so that altogether some twenty-four insects, representing eight orders, are dependent for their existence on what to the common observer appears to be nothing but “an unmeaning mass of leaves.” Among the numerous insects parasitic on the inhabitants of galls are hymenopterous flies of the family Proctotrypidae, and of the family Chalcididae, e.g. Callimome regius, the larva of which preys on the larvae of both Cynips glutinosa and its lodger Synergus facialis. The oak-apple often contains the larvae of Braconidae and Ichneumonidae, which Von Schlechtendal (loc. sup. cit. p. 33) considers to be parasites not on the owner of the gall, Andricus terminalis, but on inquilinous Tortricidae. Birds are to be included among the enemies of gall-insects. Oak-galls, for example, are broken open by the titmouse in order to obtain the grub within, and the “button-galls” of Neuroterus numismatis, Oliv., are eaten by pheasants.
A great variety of deformations and growths produced by insects and mites as well as by fungi have been described. They are in some cases very slight, and in others form remarkably large and definite structures. The whole are now included under the term Cecidia; a prefix gives the name of the organism to which the attacks are due, e.g. Phytoptocecidia are the galls formed by Phytoptid mites. Simple galls are those that arise when only one member of a plant is involved; compound galls are the result of attacks on buds. Amongst the most remarkable galls recently discovered we may mention those found on Eucalyptus, Casuarina and other trees and plants in Australia. They are remarkable for their variety, and are due to small scale-insects of the peculiar sub-family Brachyscelinae. As regards the mode of production of galls, the most important distinction is between galls that result from the introduction of an egg, or other matter, into the interior of the plant, and those that are due to an agent acting externally, the gall in the latter case frequently growing in such a manner as ultimately to enclose its producers. The form and nature of the gall are the result of the powers of growth possessed by the plant. It has long been known, and is now generally recognized, that a gall can only be produced when the tissue of a plant is interfered with during, or prior to, the actual development of the tissue. Little more than this is known. The power that gall-producers possess of influencing by direct interference the growth of the cells of the plant that affords them the means of subsistence is an art that appears to be widely spread among animals, but is at the same time one of which we have little knowledge. The views of Adler as to the alternation of generations of numerous gall-flies have been fully confirmed, it having been ascertained by direct observation that the galls and the insects produced from them in one generation are entirely different from the next generation; and it has also been rendered certain that frequently one of the alternate generations is parthenogenetic, no males being produced. It is supposed that these remarkable phenomena have gradually been evoked by difference in the nutrition of the alternating generations. When two different generations are produced in one year on the same kind of tree it is clear the properties of the sap and tissues of the tree must be diverse so that the two generations are adapted to different conditions. In some cases the alternating generations are produced on different species of trees, and even on different parts of the two species.
On galls and their makers and inhabitants see further—J.T.C. Ratzeburg, Die Forst-Insecten, Teil iii. pp. 53 seq. (Berlin, 1844); T.W. Harris, Insects injurious to Vegetation (Boston, U.S., 2nd ed., 1852); C.L. Koch, Die Pflanzenläuse Aphiden (Nuremberg, 1854); T. Hartig, Die Familien der Blattwespen und Holzwespen (Berlin, 1860); Walsh, “On the Insects, Coleopterous, Hymenopterous and Dipterous, inhabiting the Galls of certain species of Willow,” Proc. Ent. Soc. Philadelphia, iii. (1863-1864), pp. 543-644, and vi. (1866-1867), pp. 223-288; T.A. Marshall, “On some British Cynipidae,” Ent. Month. Mag. iv. pp. 6-8, &c.; H.W. Kidd and Albert Müller, “A List of Gall-bearing British Plants,” ib. v. pp. 118 and 216; G.L. Mayr, Die mitteleuropäischen Eichengallen in Wort und Bild (Vienna, 1870-1871), and the translation of that work, with notes, in the Entomologist, vols. vii. seq.; also, by the same author, “Die Einmiethler der mitteleuropäischen Eichengallen,” Verhandl. d. zoolog.-bot. Ges. in Wien, xxii. pp. 669-726; and “Die europäischen Torymiden,” ib. xxiv. pp. 53-142 (abstracted in Cistula entomologica, i., London, 1869-1876); F. Löw, “Beiträge zur Kenntnis der Gallmücken,” ib. pp. 143-162, and 321-328; J.E. von Bergenstamm and P. Löw, “Synopsis Cecidomyidarum,” ib. xxvi. pp. 1-104; Perris, Ann. Soc. Entom. de France, 4th ser. vol. x. pp. 176-185; R. Osten-Sacken, “On the North American Cecidomyidae,” Smithsonian Miscellaneous Collections, vol. vi. (1867), p. 173; E.L. Taschenberg, Entomologie für Gärtner und Gartenfreunde (Leipzig, 1871); J.W.H. Traill, “Scottish Galls,” Scottish Naturalist, i. (1871), pp. 123, &c.; Albert Müller, “British Gall Insects,” The Entomologist’s Annual for 1872, pp. 1-22; B. Altum, Forstzoologie, iii. “Insecten,” pp. 250 seq. (Berlin, 1874); J.H. Kaltenbach, Die Pflanzenfeinde aus der Classe der Insecten (Stuttgart, 1874); A. d’Arbois de Jubainville and J. Vesque, Les Maladies des plantes cultivées, pp. 98-105 (Paris, 1878).
1 Quoted in Zoological Record, iv. (1867), p. 192.
2 P. Cameron, Scottish Naturalist, ii. pp. 11-15.
3 Entomologist, vii. p. 47.
4 See in Proc. Entom. Soc. of London for the Year 1873, p. xvi.
5 See A. Müller, Gardener’s Chronicle (1871), pp. 1162 and 1518; and E.A. Fitch, Entomologist, xi. p. 129.
6 Entomologist, vi. pp. 275-278, 339-340.
7 Verhandl. d. zoolog.-bot. Ges. in Wien, xxi. p. 799.
8 Darwin, Variations of Animals and Plants under Domestication, ii. p. 282.
9 “Recherches pour servir à l’histoire des galles,” Ann. des sci. nat. xix. pp. 293 sqq.
10 According to Dr Adler, alternation of generations takes place between N. lenticularis and Spathegaster baccarum (see E.A. Ormerod, Entomologist, xi. p. 34).
11 See Westwood, Introd. to the Mod. Classif. of Insects, ii. (1840) p. 130.
12 For figures and descriptions of insect and gall, see Entomologist, iv. p. 17, vii. p. 241, ix. p. 53, xi. p. 131.
13 Scottish Naturalist, i. (1871) p. 116, &c.
14 Vinen, Journ. de pharm. et de chim. xxx. (1856) p. 290; “English Ink-Galls,” Pharm. Journ. 2nd ser. iv. p. 520.
15 See Pereira, Materia Medica, vol. ii. pt. i. p. 347; Pharm. Journ. 1st ser. vol. viii. pp. 422-424.
16 See R.H. Stretch and C.D. Gibbes, Proc. California Acad. of Sciences, iv. pp. 265 and 266.
17 A Complete History of Drugs (translation), p. 169 (London, 1748).
18 F. Porter Smith, Contrib. towards the Mat. Medica ... of China, p. 100 (1871).
19 R.F. Burton, First Footsteps in E. Africa, p. 178 (1856).
20 A.S. Packard, jun., Guide to the Study of Insects, p. 205 (Salem, 1870).
21 On the Cecidomyids of Quercus Cerris, see Fitch, Entomologist, xi. p. 14.
22 See, on Cecidomyia oenephila, Von Haimhoffen, Verhandl. d. zoolog.-bot. Ges. in Wien, xxv. pp. 801-810.
23 See Entomologist’s Month. Mag. iv. (1868) p. 233; and for figure and description, Entomologist, xi. p. 13.
24 A.S. Packard, jun., Our Common Insects, p. 203 (Salem, U.S. 1873). On the Hessian fly, Cecidomyia destructor, Say, the May brood of which produces swellings immediately above the joints of barley attacked by it, see Asa Fitch, The Hessian Fly (Albany, 1847), reprinted from Trans. New York State Agric. Soc. vol. vi.
25 J. Winnertz, Beitrag zu einer Monographie der Sciarinen, p. 164 (Vienna, 1867).
26 Asa Fitch, First and Second Rep. on the Noxious ... Insects of the State of New York, p. 167 (Albany, 1856).
27 See E. Doubleday, Pharm. Journ. 1st ser, vol. vii. p. 310: and Pereira, ib. vol. iii. p. 377.
28 Dingler’s Polyt. Journ. ccxvi. p. 453.
29 For figure and description see Zoology of the “Erebus” and “Terror,” ii. pp. 46, 47 (1844-1875).
30 On the mite-galls and their makers, see F. Löw, “Beiträge zur Naturgesch. der Gallmilben (Phytoptus, Duj.),” Verhandl. d. zoolog.-bot. Ges. in Wien, xxiv. (1874), pp. 2-16, with plate; and “Über Milbengallen (Acarocecidien) der Wiener-Gegend,” ib. pp. 495-508; Andrew Murray, Economic Entomology, Aptera, pp. 331-374 (1876); and F.A.W. Thomas, Ältere und neue Beobachtungen über Phytopto-Cecidien (Halle, 1877).


