Fuel (O. Fr. feuaile, popular Lat. focalia, from focus, hearth, fire), a term applicable to all substances that can be usefully employed for the production of heat by combustion. Any element or combination of elements susceptible of oxidation may under appropriate conditions be made to burn; but only those that ignite at a moderate initial temperature and burn with comparative rapidity, and, what is practically of more importance, are obtainable in quantity at moderate prices, can fairly be regarded as fuels. The elementary substances that can be so classed are primarily hydrogen, carbon and sulphur, while others finding more special applications are silicon, phosphorus, and the more readily oxidizable metals, such as iron, manganese, aluminium and magnesium. More important, however, than the elements are the carbohydrates or compounds of carbon, oxygen and hydrogen, which form the bulk of the natural fuels, wood, peat and coal, as well as of their liquid and gaseous derivatives—coal-gas, coal-tar, pitch, oil, &c., which have high values as fuel. Carbon in the elementary form has its nearest representative in the carbonized fuels, charcoal from wood and coke from coal.
Solid Fuels.
Wood may be considered as having the following average composition when in the air-dried state: Carbon, 39.6; hydrogen, 4.8; oxygen, 34.8; ash, 1.0; water, 20%. When it is freshly felled, the water may be from 18 to Wood. 50%. Air-dried or even green wood ignites readily when a considerable surface is exposed to the kindling flame, but in large masses with regular or smooth surfaces it is often difficult to get it to burn. When previously torrefied or scorched by heating to a temperature of about 200°, at which incipient charring is set up, it is exceedingly inflammable. The ends of imperfectly charred boughs from the charcoal heaps in this condition are used in Paris and other large towns in France for kindling purposes, under the name of fumerons. The inflammability, however, varies with the density,—the so-called hard woods, oak, beech and maple, taking fire less readily than the softer, and, more especially, the coniferous varieties rich in resin. The calorific power of absolutely dry woods may as an average be taken at about 4000 units, and when air-dried, i.e. containing 25% of water, at 2800 to 3000 units. Their evaporative values, i.e. the quantities of water evaporated by unit weight, are 3.68 and 4.44.
Wood being essentially a flaming fuel is admirably adapted for use with heat-receiving surfaces of large extent, such as locomotive and marine boilers, and is also very clean in use. The absence of all cohesion in the cinders or unburnt carbonized residue causes a large amount of ignited particles to be projected from the chimney, when a rapid draught is used, unless special spark-catchers of wire gauze or some analogous contrivance are used. When burnt in open fireplaces the volatile products given off in the apartment on the first heating have an acrid penetrating odour, which is, however, very generally considered to be agreeable. Owing to the large amount of water present, no very high temperatures can be obtained by the direct combustion of wood, and to produce these for metallurgical purposes it is necessary to convert it previously either into charcoal or into inflammable gas.
Peat includes a great number of substances of very unequal fuel value, the most recently formed spongy light brown kind approximating in composition to wood, while the dense pitchy brown compact substance, obtained from Peat. the bottom of bogs of ancient formation, may be compared with lignite or even in some instances with coal. Unlike wood, however, it contains incombustible matter in variable but large quantity, from 5 to 15% or even more. Much of this, when the amount is large, is often due to sand mechanically intermixed; when air-dried the proportion of water is from 8 to 20%. When these constituents are deducted the average composition may be stated to be—carbon, 52 to 66; hydrogen, 4.7 to 7.4; oxygen, 28 to 39; and nitrogen, 1.5 to 3%. Average air-dried peat may be taken as having a calorific value of 3000 to 3500 units, and when dried at 100° C., and with a minimum of ash (4 to 5%), at about 5200 units, or from a quarter to one-third more than that of an equal weight of wood. The lighter and more spongy varieties of peat when air-dried are exceedingly inflammable, firing at a temperature of 200° C.; the denser pulpy kinds ignite less readily when in the natural state, and often require a still higher temperature when prepared by pulping and compression or partial carbonization. Most kinds burn with a red smoky flame, developing a very strong odour, which, however, has its admirers in the same way that wood smoke has. This arises from the destructive distillation of imperfectly carbonized organic matter. The ash, like that of wood, is light and powdery, except when much sand is present, when it is of a denser character.
Peat is principally found in high latitudes, on exposed high tablelands and treeless areas in more temperate climates, and in the valleys of slow-flowing rivers,—as in Ireland, the west of Scotland, the tableland of Bavaria, the North German plain, and parts of the valleys of the Somme, Oise and a few other rivers in northern France. A principal objection to its use is its extreme bulk, which for equal evaporative effect is from 8 to 18 times that of coal. Various methods have been proposed, and adopted more or less successfully, for the purpose of increasing the density of raw peat by compression, either with or without pulping; the latter process gives the heaviest products, but the improvement is scarcely sufficient to compensate for the cost.
Lignite or brown coal is of intermediate character between peat and coal proper. The best kinds are undistinguishable in quality from free-burning coals, and the lowest earthy kinds are not equal to average peat. When freshly Lignite. raised, the proportion of water may be from 45 to 50% and even more, which is reduced from 28 to 20% by exposure to dry air. Most varieties, however, when fully dried, break up into powder, which considerably diminishes their utility as fuel, as they cannot be consolidated by coking. Lignite dust may, however, be compacted into serviceable blocks for burning, by pressure in machines similar to those used for brickmaking, either in the wet state as raised from the mines or when kiln-dried at 200° C. This method was adopted to a very large extent in Prussian Saxony. The calorific value varies between 3500 and 5000 units, and the evaporative factor from 2.16 when freshly raised to 5.84 for the best kinds of lignite when perfectly dried.
Of the other natural fuels, apart from coal (q.v.), the most important is so-called vegetable refuse, such as cotton stalks, brushwood, straw, and the woody residue of sugar-cane after the extraction of the saccharine juice known as Other natural fuels. megasse or cane trash. These are extensively used in countries where wood and coal are scarce, usually for providing steam in the manufactures where they arise, e.g. straw for thrashing, cotton stalks for ploughing, irrigating, or working presses, and cane trash for boiling down sugar or driving the cane mill. According to J. Head (Proc. Inst. of Civil Engineers, vol. xlviii. p. 75), the evaporative values of 1 ℔ of these different articles when burnt in a tubular boiler are—coal, 8 ℔; dry peat, 4 ℔; dry wood, 3.58-3.52 ℔; cotton stalks or megasse, 3.2-2.7 ℔; straw, 2.46-2.30 ℔. Owing to the siliceous nature of the ash of straw, it is desirable to have a means of clearing the grate bars from slags and clinkers at short intervals, and to use a steam jet to clear the tubes from similar deposits.
The common fuel of India and Egypt is derived from the dung of camels and oxen, moulded into thin cakes, and dried in the sun. It has a very low heating power, and in burning gives off acrid ammoniacal smoke and vapour.
Somewhat similar are the tan cakes made from spent tanners’ bark, which are used to some extent in eastern France and in Germany. They are made by moulding the spent bark into cakes, which are then slowly dried by exposure to the air. Their effect is about equivalent to 80 and 30% of equal weights of wood and coal respectively.
Sulphur, phosphorus and silicon, the other principal combustible elements, are only of limited application as fuels. The first is used in the liquidation of sulphur-bearing rocks. The ore is piled into large heaps, which are ignited at the bottom, a certain proportion, from one-fourth to one-third, of the sulphur content being sacrificed, in order to raise the mass to a sufficient temperature to allow the remainder to melt and run down to the collecting basin. Another application is in the so-called “pyritic smelting,” where ores of copper (q.v.) containing iron pyrites, FeS2, are smelted with appropriate fluxes in a hot blast, without preliminary roasting, the sulphur and iron of the pyrites giving sufficient heat by oxidation to liquefy both slag and metal. Phosphorus, which is of value from its low igniting point, receives its only application in the manufacture of lucifer matches. The high temperature produced by burning phosphorus is in part due to the product of combustion (phosphoric acid) being solid, and therefore there is less heat absorbed than would be the case with a gaseous product. The same effect is observed in a still more striking manner with silicon, which in the only special case of its application to the production of heat, namely, in the Bessemer process of steel-making, gives rise to an enormous increase of temperature in the metal, sufficient indeed to keep the iron melted. The absolute calorific value of silicon is lower than that of carbon, but the product of combustion (silica) being non-volatile at all furnace temperatures, the whole of the heat developed is available for heating the molten iron, instead of a considerable part being consumed in the work of volatilization, as is the case with carbonic oxide, which burns to waste in the air.
Assay and Valuation of Carbonaceous Fuels.—The utility or value of a fuel depends upon two principal factors, namely, its calorific power and its calorific intensity or pyrometric effect, that is, the sensible temperature of the products of combustion. Calorific power. The first of these is constant for any particular product of combustion independently of the method by which the burning is effected, whether by oxygen, air or a reducible metallic oxide. It is most conveniently determined in the laboratory by measuring the heat evolved during the combustion of a given weight of the fuel. The method of Lewis Thompson is one of the most useful. The calorimeter consists of a copper cylinder in which a weighed quantity of coal intimately mixed with 10-12 parts of a mixture of 3 parts of potassium chlorate and 1 of potassium nitrate is deflagrated under a copper case like a diving-bell, placed at the bottom of a deep glass jar filled with a known weight of water. The mixture is fired by a fuse of lamp-cotton previously soaked in a nitre solution and dried. The gases produced by the combustion rising through the water are cooled, with a corresponding increase of temperature in the latter, so that the difference between the temperature observed before and after the experiment measures the heat evolved. The instrument is so constructed that 30 grains (2 grammes) of coal are burnt in 29,010 grains of water, or in the proportion of 1 to 937, these numbers being selected that the observed rise of temperature in Fahrenheit degrees corresponds to the required evaporative value in pounds, subject only to a correction for the amount of heat absorbed by the mass of the instrument, for which a special coefficient is required and must be experimentally determined. The ordinary bomb calorimeter is also used. An approximate method is based upon the reduction of lead oxide by the carbon and hydrogen of the coal, the amount of lead reduced affording a measure of the oxygen expended, whence the heating power may be calculated, 1 part of pure carbon being capable of producing 34½ times its weight of lead. The operation is performed by mixing the weighed sample with a large excess of litharge in a crucible, and exposing it to a bright red heat for a short time. After cooling, the crucible is broken and the reduced button of lead is cleaned and weighed. The results obtained by this method are less accurate with coals containing much disposable hydrogen and iron pyrites than with those approximating to anthracite, as the heat equivalent of the hydrogen in excess of that required to form water with the oxygen of the coal is calculated as carbon, while it is really about four times as great. Sulphur in iron pyrites also acts as a reducing agent upon litharge, and increases the apparent effect in a similar manner.
The evaporative power of a coal found by the above methods, and also by calculating the separate calorific factors of the components as determined by the chemical analysis, is always considerably above that obtained by actual combustion under a steam boiler, as in the latter case numerous sources of loss, such as imperfect combustion of gases, loss of unburnt coal in cinders, &c., come into play, which cannot be allowed for in laboratory experiments. It is usual, therefore, to determine the value of a coal by the combustion of a weighed quantity in the furnace of a boiler, and measuring the amount of water evaporated by the heat developed.
In a research upon the heating power and other properties of coal for naval use, carried out by the German admiralty, the results tabulated below were obtained with coals from different localities.
| Slag left in Grate. |
Ashes in Ashpit. |
Soot in Flues. |
Water evaporated by 1 ℔ of Coal |
|
| Westphalian gas coals | 0.33-6.42 | 2.83-6.53 | 0.32-0.46 | 6.60-7.45 ℔ |
| Do. bituminous coals | 0.98-9.10 | 1.97-9.63 | 0.24-0.88 | 7.30-8.66 |
| Do. dry coals | 1.93-5.70 | 4.37-10.63 | 0.24-0.48 | 7.03-8.51 |
| Silesian coals | 0.92-1.30 | 3.15-3.50 | 0.24-0.30 | 6.73-7.10 |
| Welsh steam coals | 1.20-4.07 | 4.07 | 0.32 | 8.41 |
| Newcastle coals | 1.92 | 2.57 | 0.35 | 7.28 |
The heats of combustion of elements and compounds will be found in most of the larger works on physical and chemical constants; a convenient series is given in the Annuaire du Bureau des Longitudes, appearing in alternate years. The following figures for the principal fuel elements are taken from the issue for 1908; they are expressed in gramme “calories” or heat units, signifying the weight of water in grammes that can be raised 1° C. in temperature by the combustion of 1 gramme of the substance, when it is oxidized to the condition shown in the second column:
| Element. | Product of Combustion. | Calories. |
| Hydrogen | Water, H2O, condensed to liquid | 34,500 |
| ” as vapour | 29,650 | |
| Carbon— | ||
| Diamond | Carbon Dioxide, CO2 | 7,868 |
| Graphite | ” ” | 7,900 |
| Amorphous | ” ” | 8,133 |
| Silicon— | ||
| Amorphous | Silicon Dioxide, SiO2 | 6,414 |
| Crystallized | ” ” | 6,570 |
| Phosphorus | Phosphoric pentoxide, P2O5 | 5,958 |
| Sulphur | Sulphur dioxide, SO2, gaseous | 2,165 |
The results may also be expressed in terms of the atomic equivalent of the combustible by multiplying the above values by the atomic weight of the substance, 12 for carbon, 28 for silicon, &c.
In all fuels containing hydrogen the calorific value as found by the calorimeter is higher than that obtainable under working conditions by an amount equal to the latent heat of volatilization of water which reappears as heat when the vapour is condensed, though under ordinary conditions of use the vapour passes away uncondensed. This gives rise to the distinction of higher and lower calorific values for such substances, the latter being those generally used in practice. The differences for the more important compound gaseous fuels are as follows:—
| Calorific Value. | ||
| Higher. | Lower. | |
| Acetylene, C2H2 | 11,920 | 11,500 |
| Ethylene, C2H4 | 11,880 | 11,120 |
| Methane, CH4 | 13,240 | 11,910 |
| Carbon monoxide, CO | 2,440 | 2,440 |
The calorific intensity or pyrometric effect of any particular fuel depends upon so many variable elements that it cannot be determined except by actual experiment. The older method was to multiply the weight of the products of combustion Caloric intensity. by their specific heats, but this gave untrustworthy results as a rule, on account of two circumstances—the great increase in specific heat at high temperatures in compound gases such as water and carbon dioxide, and their instability when heated to 1800° or 2000°. At such temperatures dissociation to a notable extent takes place, especially with the latter substance, which is also readily reduced to carbon monoxide when brought in contact with carbon at a red heat—a change which is attended with a large heat absorption. This effect is higher with soft kinds of carbon, such as charcoal or soft coke, than with dense coke, gas retort carbon or graphite. These latter substances, therefore, are used when an intense local heat is required, as for example, in the Deville furnace, to which air is supplied under pressure. Such a method is, however, only of very special application, the ordinary method being to supply air to the fire in excess of that required to burn the fuel to prevent the reduction of the carbon dioxide. The volume of flame, however, is increased by inert gas, and there is a proportionate diminution of the heating effect. Under the most favourable conditions, when the air employed has been previously raised to a high temperature and pressure, the highest attainable flame temperature from carbonaceous fuel seems to be about 2100°-2300° C.; this is realized in the bright spots or “eyes” of the tuyeres of blast furnaces.
Very much higher temperatures may be reached when the products of combustion are not volatile, and the operation can be effected by using the fuel and oxidizing agent in the proportions exactly required for perfect combustion and intimately mixed. These conditions are met in the “Thermit” process of Goldschmidt, where finely divided aluminium is oxidized by the oxide of some similar metal, such as iron, manganese or chromium, the reaction being started by a primer of magnesium and barium peroxide. The reaction is so rapidly effected that there is an enormous rise in temperature, estimated to be 5400° F. (3000° C.), which is sufficient to melt the most refractory metals, such as chromium. The slag consists of alumina which crystallizes in the forms of corundum and ruby, and is utilized as an abrasive under the name of corubin.
The chemical examination includes the determination of (1) moisture, (2) ash, (3) coke, (4) volatile matter, (5) fixed carbon in coke, (6) sulphur, (7) chlorine, (8) phosphorus. Moisture is determined by noting the loss in weight when a sample is heated at 100° for about one hour. The ash is determined by heating a sample in a muffle furnace until all the combustible matter has been burnt off. The ash, which generally contains silica, oxides of the alkaline earths, ferric oxide (which gives the ash a red colour), sulphur, &c., is analysed by the ordinary gravimetric methods. The determination of coke is very important on account of the conclusions concerning the nature of the coal which it permits to be drawn. A sample is finely powdered and placed in a covered porcelain crucible, which is surrounded by an outer one, the space between them being packed with small coke. The crucibles are heated in a wind furnace for 1 to 1½ hours, then allowed to cool, the inner crucible removed, and the coke weighed. The coke may be (1) pulverulent, (2) slightly fritted, (3) spongy and swelled, (4) compact. Pulverulent cokes indicate a non-caking bituminous coal, rich in oxygen if the amount be below 60%, but if the amount be very much less it generally indicates a lignite; if the amount be above 80% it indicates an anthracite containing little oxygen or hydrogen. A fritted coke indicates a slightly coking coal, while the spongy appearance points to a highly coking coal which has been partly fused in the furnace. A compact coke is yielded by good coking coals, and is usually large in amount. The volatile matters are determined as the loss of weight on coking less the amount of moisture. The “fixed carbon” is the carbon retained in the coke, which contains in addition the ash already determined. The fixed carbon is therefore the difference between the coke and the ash, and may be determined from these figures; or it may be determined directly by burning off the coke in a muffle and noting the loss in weight. Sulphur may be present as (1) organic sulphur, (2) as iron pyrites or other sulphides, (3) as the sulphates of calcium, aluminium and other metals; but the amount is generally so small that only the total sulphur is determined. This is effected by heating a mixture of the fuel with lime and sodium carbonate in a porcelain dish to redness in a muffle until all the carbonaceous matter has been burnt off. The residue, which contains the sulphur as calcium sulphate, is transferred to a beaker containing water to which a little bromine has been added. Hydrochloric acid is carefully added, the liquid filtered and the residue washed. To the filtrate ammonia is added, and then barium chloride, which precipitates the sulphur as barium sulphate. Sulphur existing in the form of sulphates may be removed by washing a sample with boiling water and determining the sulphuric acid in the solution. The washed sample is then fused in the usual way to determine the proportion of sulphur existing as iron pyrites. The distinction between sulphur present as sulphate and sulphide is of importance in the examination of coals intended for iron smelting, as the sulphates of the earthy metals are reduced by the gases of the furnace to sulphides, which pass into the slag without affecting the quality of the iron produced, while the sulphur of the metallic sulphides in the ash acts prejudicially upon the metal. Coals for gas-making should contain little sulphur, as the gases produced in the combustion are noxious and have very corrosive properties. Chlorine is rarely determined, but when present in quantity it corrodes copper and brass boiler tubes, with which consequently chlorine-bearing coals cannot be used. The element is determined by fusing with soda lime in a muffle, dissolving the residue in water and precipitating with silver nitrate. Phosphorus is determined in the ash by fusing it with a mixture of sodium and potassium carbonates, extracting the residue with hydrochloric acid, and twice evaporating to dryness with the same acid. The residue is dissolved in hydrochloric acid, a few drops of ferric chloride added, and then ammonia in excess. The precipitate of ferric phosphate is then treated as in the ordinary estimation of phosphates. If it be necessary to determine the absolute amount of carbon and hydrogen in a fuel, the dried sample is treated with copper oxide as in the ordinary estimation of these elements in organic compounds.
Liquid Fuel.
Vegetable oil is not used for fuel except for laboratory purposes, partly because its constituent parts are less adaptable for combustion under the conditions necessary for steam-raising, but chiefly because of the commercial difficulty of producing it with sufficient economy to compete with mineral fuel either solid or liquid.
The use of petroleum as fuel had long been recognized as a scientific possibility, and some attempts had been made to adopt it in practice upon a commercial scale, but the insufficiency, and still more the irregularity, of the supplies prevented it from coming into practical use to any important extent until about 1898, when discoveries of oil specially adapted by chemical composition for fuel purposes changed the aspect of the situation. These discoveries of special oil were made first in Borneo and later in Texas, and experience in treating the oils from both localities has shown that while not less adapted to produce kerosene or illuminating oil, they are better adapted to produce fuel oil than either the Russian or the Pennsylvanian products. Texas oil did not hold its place in the market for long, because the influx of water into the wells lowered their yield, but discoveries of fuel oil in Mexico have come later and will help to maintain the balance of the world’s supply, although this is still a mere fraction of the assured supply of coal.
With regard to the chemical properties of petroleum, it is not necessary to say more in the present place than that the lighter and more volatile constituents, known commercially as naphtha and benzene, must be removed by distillation in order to leave a residue composed principally of hydrocarbons which, while containing the necessary carbon for combustion, shall be sufficiently free from volatile qualities to avoid premature ignition and consequent danger of explosion. Attempts have been made to use crude oil for fuel purposes, and these have had some success in the neighbourhood of the oil wells and under boilers of unusually good ventilation both as regards their chimneys and the surroundings of their stokeholds; but for reasons both of commerce and of safety it is not desirable to use crude oil where some distillation is possible. The more complete the process of distillation, and the consequent removal of the volatile constituents, the higher the flash-point, and the more turgid and viscous is the fuel resulting; and if the process is carried to an extreme, the residue or fuel becomes difficult to ignite by the ordinary process of spraying or atomizing mechanically at the moment immediately preceding combustion. The proportions which have been found to work efficiently in practice are as follows:—
| Carbon | 88.00 % |
| Hydrogen | 10.75 % |
| Oxygen | 1.25 % |
| ——— | |
| Total | 100 |
The standards of safety for liquid fuel as determined by flash-point are not yet finally settled, and are changing from time to time. The British admiralty require a flash-point of 270° F., and to this high standard, and the consequent viscosity of the fuel used by vessels in the British fleet, may partly be attributed the low rate of combustion that was at first found possible in them. The German admiralty have fixed a flash-point of 187° F., and have used oil of this standard with perfect safety, and at the same time with much higher measure of evaporative duty than has been attained in British war-vessels. In the British mercantile marine Lloyd’s Register has permitted fuel with a flash-point as low as 150° F. as a minimum, and no harm has resulted. The British Board of Trade, the department of the government which controls the safety of passenger vessels, has fixed a higher standard upon the basis of a minimum of 185°. In the case of locomotives the flash-point as a standard of safety is of less importance than in the case of stationary or marine boilers, because the storage is more open, and the ventilation, both of the storage tanks and the boilers during combustion, much more perfect than in any other class of steam-boilers.
The process of refining by distillation is also necessary to reduce two impurities which greatly retard storage and combustion, i.e. water and sulphur. Water is found in all crude petroleum as it issues from the wells, and sulphur exists in important quantities in oil from the Texas wells. Its removal was at first found very expensive, but there no longer exists difficulty in this respect, and large quantities of petroleum fuel practically free from sulphur are now regularly exported from Texas to New York and to Europe.
Water mixed with fuel is in intimate mechanical relation, and frequently so remains in considerable quantities even after the process of distillation. It is in fact so thoroughly mixed as to form an emulsion. The effect of feeding such a mixture into a furnace is extremely injurious, because the water must be decomposed chemically into its constituents, hydrogen and oxygen, thus absorbing a large quantity of heat which would otherwise be utilized for evaporation. Water also directly delays combustion by producing from the jet a long, dull, red flame instead of a short bright, white flame, and the process of combustion, which should take place by vaporization of the oil near the furnace mouth, is postponed and transferred to the upper part of the combustion-box, the tubes, and even the base of the chimney, producing loss of heat and injury to the boiler structure. The most effective means of ridding the fuel of this dangerous impurity is by heat and settlement. The coefficients of expansion of water and oil by heat are substantially different, and a moderate rise of temperature therefore separates the particles and precipitates the water, which is easily drawn off—leaving the oil available for use. The heating and precipitation are usually performed upon a patented system of settling tanks and heating apparatus known as the Flannery-Boyd system, which has proved itself indispensable for the successful use at sea of petroleum fuel containing any large proportion of water.
The laboratory and mechanical use of petroleum for fuel has already been referred to, but it was not until the year 1870 that petroleum was applied upon a wider and commercial scale. In the course of distillation of Russian crude Progress of liquid fuel. petroleum for the production of kerosene or lamp oil, large quantities of refuse were produced—known by the Russian name of astatki—and these were found an incumbrance and useless for any commercial purpose. To a Russian oil-refiner gifted with mechanical instinct and the genius for invention occurred the idea of utilizing the waste product as fuel by spraying or atomizing it with steam, so that, the thick and sluggish fluid being broken up into particles, the air necessary for combustion could have free access to it. The earliest apparatus for this purpose was a simple piece of gas-tube, into which the thick oil was fed; by another connexion steam at high pressure was admitted to an inner and smaller tube, and, the end of the tube nearest to the furnace being open, the pressure of the steam blew the oil into the furnace, and by its velocity broke it up into spray. The apparatus worked with success from the first. Experience pointed out the proper proportionate sizes for the inlets of steam and oil, the proper pressure for the steam, and the proportionate sizes for the orifices of admission to the furnaces, as well as the sizes of air-openings and best arrangements of fire-bricks in the furnaces themselves; and what had been a waste product now became a by-product of great value. Practically all the steam power in South Russia, both for factories and navigation of the inland seas and rivers, is now raised from astatki fuel.
In the Far East, including Burma and parts of China and Japan, the use of liquid fuel spread rapidly during the years 1899, 1900 and 1901, owing entirely to the development of the Borneo oil-fields by the enterprise of Sir Marcus Samuel and the large British corporation known as the Shell Transport and Trading Company, of which he is the head. This corporation has since amalgamated with the Royal Dutch Petroleum Company controlling the extensive wells in Dutch Borneo, and together they supply large quantities of liquid fuel for use in the Far East. In the United States of America liquid fuel is not only used for practically the whole of the manufacturing and locomotive purposes of the state of Texas, but factories in New York, and a still larger number in California, are now discarding the use of coal and adopting petroleum, because it is more economical in its consumption and also more easily handled in transit, and saves nearly all the labour of stoking. So far the supplies for China and Japan have been exported from Borneo, but the discoveries of new oil-fields in California, of a character specially adapted for fuel, have encouraged the belief that it may be possible to supply Chile and Peru and other South American countries, where coal is extremely expensive, with Californian fuel; and it has also found its way across the Pacific to Japan. There are believed to be large deposits in West Africa, but in the meantime the only sources of supply to those parts of Africa where manufacture is progressing, i.e. South Africa and Egypt, are the oil-fields of Borneo and Texas, from which the import has well begun, from Texas to Alexandria via the Mediterranean, and from Borneo to Cape Town via Singapore.
In England, notwithstanding the fact that there exist the finest coal-fields in the world, there has been a surprising development of the use of petroleum as fuel. The Great Eastern railway adapted 120 locomotive engines to its use, and these ran with regularity and success both on express passenger and goods trains until the increase in price due to short supply compelled a return to coal fuel. The London, Brighton & South Coast railway also began the adaptation of some of their locomotive engines, but discontinued the use of liquid fuel from the same cause. Several large firms of contractors and cement manufacturers, chiefly on the banks of the Thames, made the same adaptations which proved mechanically successful, but were not continued when the price of liquid fuel increased with the increased demand.
 |
| Fig. 1.—Holden Burner. |
The chief factors of economy are the greater calorific value of oil than coal (about 16 ℔ of water per ℔ of oil fuel evaporated from a temperature of 212° F.), not only in laboratory practice, but in actual use on a large scale, and the saving of labour both Economy of liquid fuel. in transit from the source of supply to the place of use and in the act of stoking the furnaces. The use of cranes, hand labour with shovels, wagons and locomotives, horses and carts, is unavoidable for the transit of coal; and labour to trim the coal, to stoke it when under combustion, and to handle the residual ashes, are all indispensable to steam-raising by coal. On the other hand, a system of pipes and pumps, and a limited quantity of skilled labour to manage them, is all that is necessary for the transit and combustion of petroleum fuel; and it is certain that even in England will be found places which, from topographical and other circumstances, will use petroleum more economically than coal as fuel for manufacturing purposes under reasonable conditions of price for the fuel.
 |
| Fig. 2.—Rusden and Eeles Burner. |
The theoretical calorific value of oil fuel is more nearly realized in practice than the theoretical calorific value of coal, because the facilities for complete combustion, due to the artificial admixture of the air by the atomizing process, are greater in the case of oil than coal, and for this reason, among others, the practical evaporative results are proportionately higher with liquid fuel. In some cases the work done in a steam-engine by 2 tons of coal has been performed by 1 ton of oil fuel, but in others the proportions have been as 3 to 2, and these latter can be safely relied on in practice as a minimum. This saving, combined with the savings of labour and transit already explained, will in the near future make the use of liquid fuel compulsory, except in places so near to coal-fields that the cost of coal becomes sufficiently low to counterbalance the savings in weight of fuel consumed and in labour in handling it. In some locomotives on the Great Eastern railway the consumption of oil and coal for the same development of horse-power was as 17 ℔ oil is to 35 ℔ coal; all, however, did not realize so high a result.
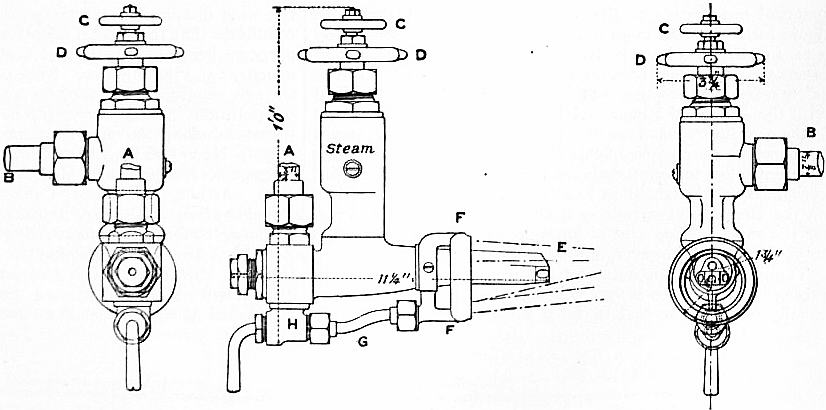
The mechanical apparatus for applying petroleum to steam-raising in locomotives is very simple. The space in the tender usually occupied by coal is closed up by steel-plating closely riveted and tested, so as to form a storage tank. From this tank Liquid fuel in locomotives. a feed-pipe is led to a burner of the combined steam-and-oil type already indicated, and this burner is so arranged as to enter a short distance inside the furnace mouth. The ordinary fire-bars are covered with a thin layer of coal, which starts the ignition in the first place, and the whole apparatus is ready for work. The burner best adapted for locomotive practice is the Holden Burner (fig. 1), which was used on the Great Eastern railway. The steam-pipe is connected at A, the oil-pipe at B, and the hand-wheels C and D are for the adjustment of the internal orifices according to the rate of combustion required. The nozzle E is directed towards the furnace, and the external ring FF, supplied by the small pipe G and the by-pass valve H, projects a series of steam jets into the furnace, independent of the injections of atomized fuel, and so induces an artificial inrush of air for the promotion of combustion. This type of burner has also been tried on stationary boilers and on board ship. It works well, although the great consumption of steam by the supplementary ring is a difficulty at sea, where the water lost by the consumption of steam cannot easily be made up.
Although the application of the new fuel for land and locomotive boilers has already been large, the practice at sea has been far more extensive. The reason is chiefly to be found in the fact that although the sources of supply are at a distance Liquid fuel at sea. from Great Britain, yet they are in countries to whose neighbourhood British steamships regularly trade, and in which British naval squadrons are regularly stationed, so that the advantages of adopting liquid fuel have been more immediate and the economy more direct. The certainty of continuous supply of the fuel and the wide distribution of storage stations have so altered the conditions that the general adoption of the new fuel for marine purposes becomes a matter of urgency for the statesman, the merchant and the engineer. None of these can afford to neglect the new conditions, lest they be noted and acted upon by their competitors. Storage for supply now exists at a number of sea ports: London, Barrow, Southampton, Amsterdam, Copenhagen, New Orleans, Savannah, New York, Philadelphia, Singapore, Hong Kong, Madras, Colombo, Suez, Hamburg, Port Arthur, Rangoon, Calcutta, Bombay, Alexandria, Bangkok, Saigon, Penang, Batavia, Surabaya, Amoy, Swatow, Fuchow, Shanghai, Hankow, Sydney, Melbourne, Adelaide, Zanzibar, Mombasa, Yokohama, Kobe and Nagasaki; also in South African and South American ports.
 |
| Fig. 3.—Storage of Liquid Fuel on Oil-carrying Steamers (Flannery-Boyd System). |
The British admiralty have undertaken experiments with liquid fuel at sea, and at the same time investigations of the possibility of supply from sources within the regions of the British empire. There is an enormous supply of shale under the north-eastern counties of England, but no oil that can be pumped—still less oil with a pressure above it so as to “gush” like the wells in America—and the only sources of liquid supply under the British flag appear to be in Burma and Trinidad. The Borneo fields are not under British control, although developed entirely by British capital. The Italian admiralty have fitted several large warships with boiler apparatus to burn petroleum. The German admiralty are regularly using liquid fuel on the China station. The Dutch navy have fitted coal fuel and liquid fuel furnaces in combination, so that the smaller powers required may be developed by coal alone, and the larger powers by supplementing coal fuel with oil fuel. The speeds of some vessels of the destroyer type have by this means been accelerated nearly two knots.
 |
| Fig. 4.—Installation on ss. “Trochas.” |
 |
| Fig. 5.—Details of Furnace, Meyer System. |
 |
| Fig. 6.—Details of Exterior Elongation of Furnace, Meyer System. |
The questions which govern the use of fuel in warships are more largely those of strategy and fighting efficiency than economy of evaporation. Indeed, the cost of constructing and maintaining in fighting efficiency a modern Advantages in warships. warship is so great that the utmost use strategically must be obtained from the vessel, and in this comparison the cost of fuel is relatively so small an item that its increase or decrease may be considered almost a negligible quantity. The desideratum in a warship is to obtain the greatest fighting efficiency based on the thickest armour, the heaviest and most numerous guns, the highest maximum speed, and, last and not least, the greatest range of effective action based upon the maximum supplies of fuel, provisions and other consumable stores that the ship can carry. Now, if by changing the type of fuel it be possible to reduce its weight by 30%, and to abolish the stokers, who are usually more than half the ship’s company, the weight saved will be represented not merely by the fuel, but by the consumable stores otherwise necessary for the stokers. Conversely, the radius of effective action of the ship will be doubled as regards consumable stores if the crew be halved, and will be increased by 50% if the same weight of fuel be carried in the form of liquid instead of coal. In space the gain by using oil fuel is still greater, and 36 cubic feet of oil as stored are equal in practical calorific value to 67 cubic feet of coal according to the allowance usual for ship’s bunkering. On the other hand, coal has been relied upon, when placed in the side bunkers of unarmoured ships, as a protection against shot and shell, and this advantage, if it really exists, could not be claimed in regard to liquid fuel.
Recent experiments in coaling warships at sea have not been very successful, as the least bad weather has prevented the safe transmission of coal bags from the collier to the ship. The same difficulty does not exist for oil fuel, which has been pumped through flexible tubing from one ship to the other even in comparatively rough weather. Smokelessness, so important a feature of sea strategy, has not always been attained by liquid fuel, but where the combustion is complete, by reason of suitable furnace arrangements and careful management, there is no smoke. The great drawback, however, to the use of liquid fuel in fast small vessels is the confined space allotted to the boilers, such confinement being unavoidable in view of the high power concentrated in a small hull. The British admiralty’s experiments, however, have gone far to solve the problem, and the quantity of oil which can be consumed by forced draught in confined boilers now more nearly equals the quantity of coal consumed under similar conditions. All recent vessels built for the British navy are so constructed that the spaces between their double bottoms are oil-tight and capable of storing liquid fuel in the tanks so formed. Most recent battleships and cruisers have also liquid fuel furnace fittings, and in 1910 it already appeared probable that the use of oil fuel in warships would rapidly develop.
In view of recent accusations of insufficiency of coal storage in foreign naval depots, by reason of the allegation that coal so stored quickly perishes, it is interesting to note that liquid fuel may be stored in tanks for an indefinite time without any deterioration whatever.
In the case of merchant steamers large progress has also been made. The Shell Transport and Trading Company have twenty-one vessels successfully navigating in all parts of the world and using liquid fuel. The Hamburg-American Advantages in merchant ships. Steamship Company have four large vessels similarly fitted for oil fuel, which, however, differ in furnace arrangements, as will be hereafter described, although using coal when the fluctuation of the market renders that the more economical fuel. One of the large American transatlantic lines is adopting liquid fuel, and French, German, Danish and American mercantile vessels are also beginning to use it in considerable amounts.
In the case of very large passenger steamers, such as those of 20 knots and upwards in the Atlantic trade, the saving in cost of fuel is trifling compared with the advantage arising from the greater weight and space available for freight. Adopting a basis of 3 to 2 as between coal consumption and oil consumption, there is an increase of 1000 tons of dead weight cargo in even a medium-sized Atlantic steamer, and a collateral gain of about 100,000 cub. ft. of measurement cargo, by reason of the ordinary bunkers being left quite free, and the oil being stored in the double bottom spaces hitherto unutilized except for the purpose of water ballast. The cleanliness and saving of time from bunkering by the use of oil fuel is also an important factor in passenger ships, whilst considerable additional speed is obtainable. The cost of the installation, however, is very considerable, as it includes not only burners and pipes for the furnaces, but also the construction of oil-tight tanks, with pumps and numerous valves and pipe connexions.
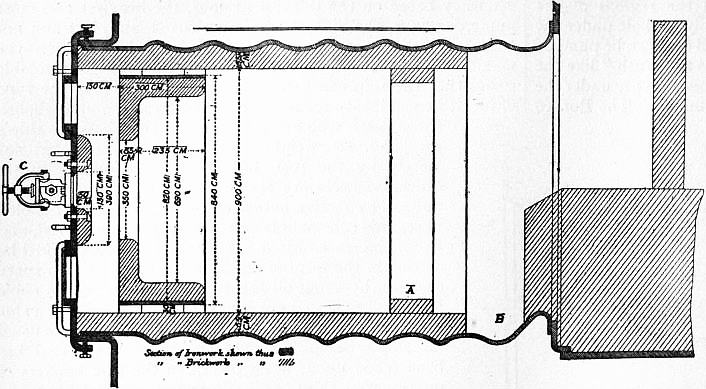 |
| Fig. 7.—Furnace on ss. “Ferdinand Laeisz.” A, it is proposed to do away with this ring of brickwork as being useless; B, it is proposed to fill this space up, thus continuing lining of furnace to combustion chamber, and also to fit protection bricks in way of saddle plate. |
 |
| Fig. 8.—Fuel Tanks, &c., of ss. “Murex.” |
 |
| Fig. 9.—Furnace Gear of ss. “Murex.” |
 |
| Fig. 10.—Section through Furnace of ss. “Murex.” |
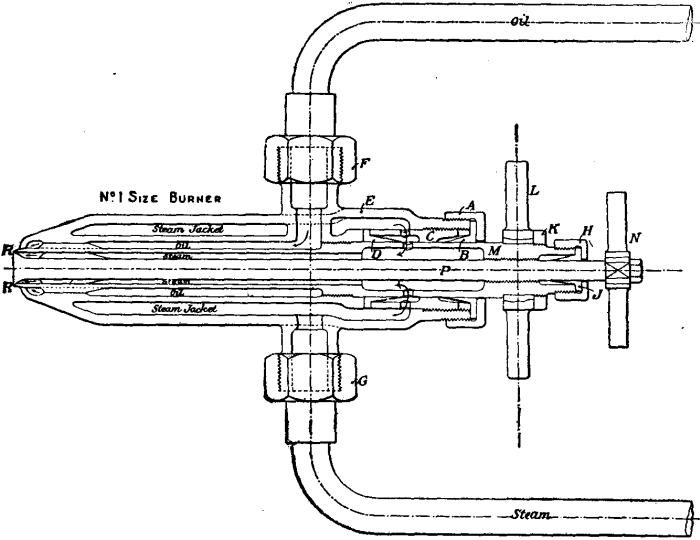
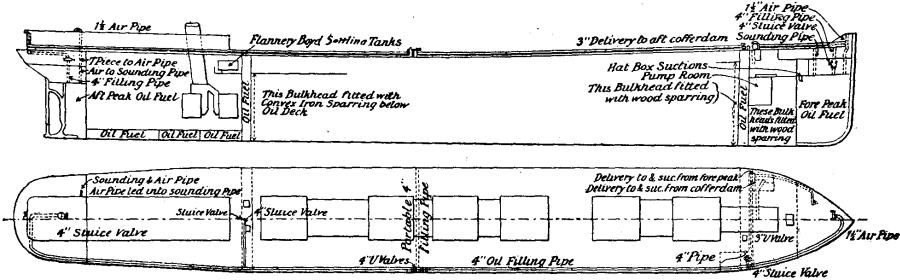
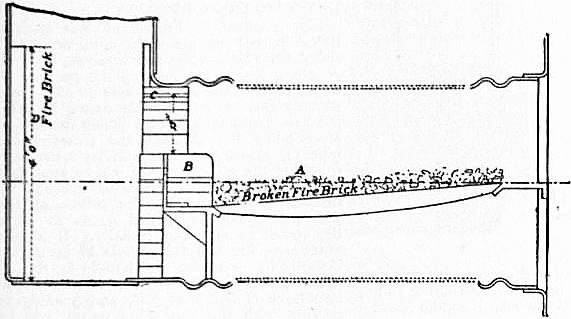
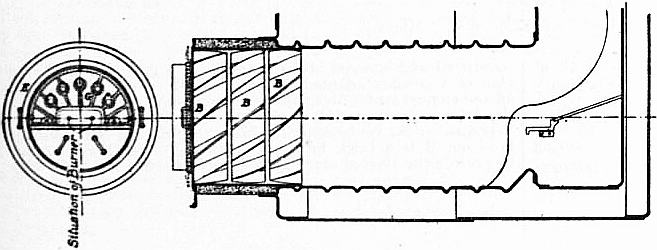
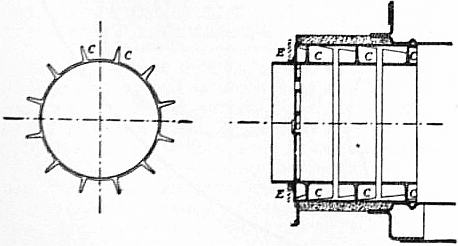
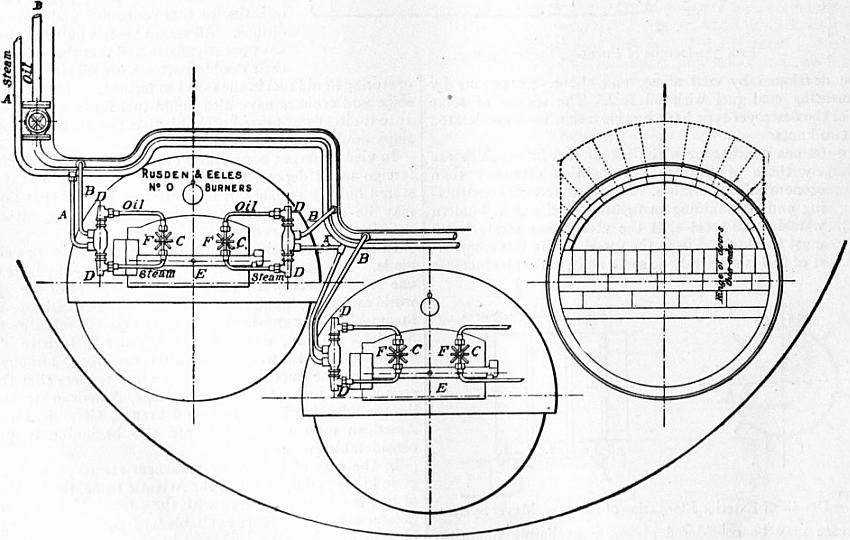
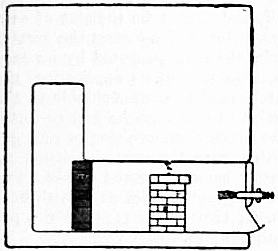
Fig. 2 shows a burner of Rusden and Eeles’ patent as generally used on board ships for the purpose of injecting the oil. A is a movable cap holding the packing B, which renders the annular spindle M oil and steam tight. E is the outer casing containing the steam jacket from which the steam, after being fed through the steam-supply pipe G, passes into the annular space surrounding the spindle P. It will be seen that if the spindle P be travelled inwards by turning the handle N, the orifice at the nozzle RR will be opened so as to allow the steam to flow out radially. If at the same time the annular spindle M be drawn inwards by revolving the handle L, the oil which passes through the supply pipe F will also have emission at RR, and, coming in contact with the outflowing steam, will be pulverized and sprayed into the furnace. Fig. 3 is a profile and plan of a steamer adapted for carrying oil in bulk, and showing all the storage arrangements for handling liquid fuel. Fig. 4 shows the interior arrangement of the boiler furnace of the steamship “Trocas.” A is broken fire-brick resting on the ordinary fire-bars, B is a brick bridge, C a casing of fire-brick intended to protect the riveted seam immediately above it from the direct impact of the flame, and D is a lining of fire-brick at the back of the combustion-box, also intended to protect the plating from the direct impact of the petroleum flame. The arrangement of the furnace on the Meyer system is shown in fig. 5, where E is an annular projection built at the mouth of the furnace, and BB are spiral passages for heating the air before it passes into the furnace. Fig. 6 shows the rings CC and details of the casting which forms the projection or exterior elongation of the furnace. The brickwork arrangement adopted for the double-ended boilers on the Hamburg-American Steamship Company’s “Ferdinand Laeisz” is represented in fig. 7. The whole furnace is lined with fire-brick, and the burner is mounted upon a circular disk plate which covers the mouth of the furnace. The oil is injected not by steam pulverization, but by pressure due to a steam-pump. The oil is heated to about 60°C. before entering the pump, and further heated to 90°C. after leaving the pump. It is then filtered, and passes to the furnace injector C at about 30-℔ pressure; and its passage through this injector and the spiral passages of which it consists pulverizes the oil into spray, in which form it readily ignites on reaching the interior of the furnace. The injector is on the Körting principle, that is, it atomizes by fracture of the liquid oil arising from its own momentum under pressure. The advantage of this system as compared with the steam-jet system is the saving of fresh water, the abstraction of which is so injurious to the boiler by the formation of scale.
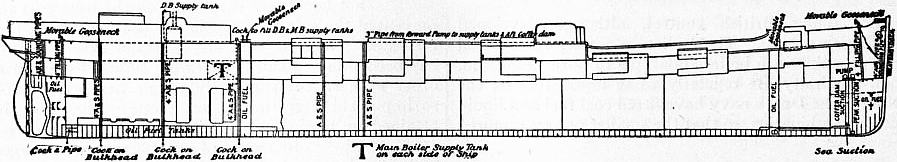
The general arrangement of the fuel tanks and filling pipes on the ss. “Murex” is shown in fig. 8; and fig. 9 represents the furnace gear of the same vessel, A being the steam-pipe, B the oil-pipe, C the injector, D the swivel upon which the injector is hung so that it may be swung clear of the furnace, E the fire-door, and F the handle for adjusting the injector. In fig. 10, which represents a section of the furnace, H is a fire-brick pier and K a fire-brick baffling bridge.
It is found in practice that to leave out the fire-bars ordinarily used for coal produces a better result with liquid fuel than the alternative system of keeping them in place and protecting them by a layer of broken fire-brick.
Boilers fitted upon all the above systems have been run for thousands of miles without trouble. In new construction it is desirable to give larger combustion chambers and longer and narrower boiler tubes than in the case of boilers intended for the combustion of coal alone.
Gaseous Fuel.
Strictly speaking, much, and sometimes even most, of the heating effected by solid or liquid fuel is actually performed by the gases given off during the combustion. We speak, however, of gaseous fuel only in those cases where we supply a combustible gas from the outset, or where we produce from ordinary solid (or liquid) fuel in one place a stream of combustible gas which is burned in another place, more or less distant from that where it has been generated.
The various descriptions of gaseous fuel employed in practice may be classified under the following heads:
I. Natural Gas.
II. Combustible Gases obtained as by-products in various technical operations.
III. Coal Gas (Illuminating Gas).
IV. Combustible Gases obtained by the partial combustion of coal, &c.
I. Natural Gas.—From time immemorial it has been known that in some parts of the Caucasus and of China large quantities of gases issue from the soil, sometimes under water, which can be lighted and burn with a luminous flame. The “eternal fires” of Baku belong to this class. In coal-mines frequently similar streams of gas issue from the coal; these are called “blowers,” and when they are of somewhat regular occurrence are sometimes conducted away in pipes and used for underground lighting. As a regular source of heating power, however, natural gas is employed only in some parts of the United States, especially in Pennsylvania, Kansas, Ohio and West Virginia, where it always occurs in the neighbourhood of coal and petroleum fields. The first public mention of it was made in 1775, but it was not till 1821 that it was turned to use at Fredonia, N.Y. In Pennsylvania natural gas was discovered in 1859, but at first very little use was made of it. Its industrial employment dates only from 1874, and became of great importance about ten years later. Nobody ever doubted that the gas found in these localities was an accumulation of many ages and that, being tapped by thousands of bore-holes, it must rapidly come to an end. This assumption was strengthened by the fact that the “gas-wells,” which at first gave out the gas at a pressure of 700 or 800, sometimes even of 1400 ℔ per sq. in., gradually showed a more and more diminishing pressure and many of them ceased to work altogether. About the year 1890 the belief was fairly general that the stock of natural gas would soon be entirely exhausted. Indeed, the value of the annual production of natural gas in the United States, computed as its equivalent of coal, was then estimated at twenty-one million dollars, in 1895 at twelve millions, in 1899 at eleven and a half millions. But the output rose again to a value of twenty-seven millions in 1901, and to fifty million dollars in 1907. Mostly the gas, derived from upwards of 10,000 gas-wells, is now artificially compressed to a pressure of 300 or 400 ℔ per sq. in. by means of steam-power or gas motors, fed by the gas itself, and is conveyed over great distances in iron pipes, from 9 or 10 to 36 in. in diameter. In 1904 nearly 30,000 m. of pipe lines were in operation. In 1907 the quantity of natural gas consumed in the United States (nearly half of which was in Pennsylvania) was 400,000 million cub. ft., or nearly 3 cub. m. Canada (Ontario) also produces some natural gas, reaching a maximum of about $746,000 in 1907.
The principal constituent of natural gas is always methane, CH4, of which it contains from 68.4 to 94.0% by volume. Those gases which contain less methane contain all the more hydrogen, viz. 2.9 to 29.8%. There is also some ethylene, ethane and carbon monoxide, rarely exceeding 2 or 3%. The quantity of incombustible gases—oxygen, carbon dioxide, nitrogen—ranges from mere traces to about 5%. The density is from 0.45 to 0.55. The heating power of 1000 cub. ft. of natural gas is equal to from 80 to 120 ℔, on the average 100 ℔, of good coal, but it is really worth much more than this proportion would indicate, as it burns completely, without smoke or ashes, and without requiring any manual labour. It is employed for all domestic and for most industrial purposes.
The origin of natural gas is not properly understood, even now. The most natural assumption is, of course, that its formation is connected with that of the petroleum always found in the same neighbourhood, the latter principally consisting of the higher-boiling aliphatic hydrocarbons of the methane series. But whence do they both come? Some bring them into connexion with the formation of coal, others with the decomposition of animal remains, others with that of diatomaceae, &c., and even an inorganic origin of both petroleum and natural gas has been assumed by chemists of the rank of D.I. Mendeléeff and H. Moissan.
II. Gases obtained as By-products.—There are two important cases in which gaseous by-products are utilized as fuel; both are intimately connected with the manufacture of iron, but in a very different way, and the gases are of very different composition.
(a) Blast-furnace Gases.—The gases issuing from the mouths of blast-furnaces (see Iron and Steel) were first utilized in 1837 by Faber du Faur, at Wasseralfingen. Their use became more extensive after 1860, and practically universal after 1870. The volume of gas given off per ton of iron made is about 158,000 cub. ft. Its percentage composition by volume is:
| Carbon monoxide | 21.6 | to | 29.0, | mostly | about | 26 | % |
| Hydrogen | 1.8 | ” | 6.3, | ” | ” | 3 | % |
| Methane | 0.1 | ” | 0.8, | ” | ” | 0.5 | % |
| Carbon dioxide | 6 | ” | 12, | ” | ” | 9.5 | % |
| Nitrogen | 51 | ” | 60, | ” | ” | 56 | % |
| Steam | 5 | ” | 12, | ” | ” | 5 | % |
| ——— | |||||||
| 100 | % | ||||||
There is always a large amount of mechanically suspended flue-dust in this gas. It is practically equal to a poor producer-gas (see below), and is everywhere used, first for heating the blast in Cowper stoves or similar apparatus, and secondly for raising all the steam required for the operation of the blast-furnace, that is, for driving the blowing-engines, hoisting the materials, &c. Where the iron ore is roasted previously to being fed into the furnace, this can also be done by this gas, but in some cases the waste in using it is so great that there is not enough left for the last purpose. The calorific power of this gas per cubic foot is from 80 to 120 B.Th.U.
Since about 1900 a great advance has been made in this field. Instead of burning the blast-furnace gas under steam boilers and employing the steam for producing mechanical energy, the gas is directly burned in gas-motors on the explosion principle. Thus upwards of three times the mechanical energy is obtained in comparison with the indirect way through the steam boiler. After all the power required for the operations of the blast-furnace has been supplied, there is a surplus of from 10 to 20 h.p. for each ton of pig-iron made, which may be applied to any other purpose.
(b) Coke-oven Gases.—Where the coking of coal is performed in the old beehive ovens or similar apparatus the gas issuing at the mouth of the ovens is lost. The attempts at utilizing the gases in such cases have not been very successful. It is quite different where coke is manufactured in the same way as illuminating gas, viz. by the destructive distillation of coal in closed apparatus (retorts), heated from the outside. This industry, which is described in detail in G. Lunge’s Coal-Tar and Ammonia (4th ed., 1909), originated in France, but has spread far more in Germany, where more than half of the coke produced is made by it; in the United Kingdom and the United States its progress has been much slower, but there also it has long been recognized as the only proper method. The output of coke is increased by about 15% in comparison with the beehive ovens, as the heat required for the process of distillation is not produced by burning part of the coal itself (as in the beehive ovens), but by burning part of the gas. The quality of the coke for iron-making is quite as good as that of beehive coke, although it differs from it in appearance. Moreover, the gases can be made to yield their ammonia, their tar, and even their benzene vapours, the value of which products sometimes exceeds that of the coke itself. And after all this there is still an excess of gas available for any other purpose.
As the principle of distilling the coal is just the same, whether the object is the manufacture of coal gas proper or of coke as the main product, although there is much difference in the details of the manufacture, it follows that the quality of the gas is very similar in both cases, so far as its heating value is concerned. Of course this heating value is less where the benzene has been extracted from coke-oven gas, since this compound is the richest heat-producer in the gas. This is, however, of minor importance in the present case, as there is only about 1% benzene in these gases.
The composition of coke-oven gases, after the extraction of the ammonia and tar, is about 53% hydrogen, 36% methane, 6% carbon monoxide, 2% ethylene and benzene, 0.5% sulphuretted hydrogen, 1.5% carbon dioxide, 1% nitrogen.
III. Coal Gas (Illuminating Gas).—Although ordinary coal gas is primarily manufactured for illuminating purposes, it is also extensively used for cooking, frequently also for heating domestic rooms, baths, &c., and to some extent also for industrial operations on a small scale, where cleanliness and exact regulation of the work are of particular importance. In chemical laboratories it is preferred to every other kind of fuel wherever it is available. The manufacture of coal gas being described elsewhere in this work (see Gas, § Manufacture), we need here only point out that it is obtained by heating bituminous coal in fireclay retorts and purifying the products of this destructive distillation by cooling, washing and other operations. The residual gas, the ordinary composition of which is given in the table below, amounts to about 10,000 cub. ft. for a ton of coal, and represents about 21% of its original heating value, 56.5% being left in the coke, 5.5% in the tar and 17% being lost. As we must deduct from the coke that quantity which is required for the heating of the retorts, and which, even when good gas producers are employed, amounts to 12% of the weight of the coal, or 10% of its heat value, the total loss of heat rises to 27%. Taking, further, into account the cost of labour, the wear and tear, and the capital interest on the plant, coal gas must always be an expensive fuel in comparison with coal itself, and cannot be thought of as a general substitute for the latter. But in many cases the greater expense of the coal gas is more than compensated by its easy distribution, the facility and cleanliness of its application, the general freedom from the mechanical loss, unavoidable in the case of coal fires, the prevention of black smoke and so forth. The following table shows the average composition of coal gas by volume and weight, together with the heat developed by its single constituents, the latter being expressed in kilogram-calories per cub. metre (0.252 kilogram-calories = 1 British heat unit; 1 cub. metre = 35.3 cub. ft.; therefore 0.1123 calories per cub. metre = 1 British heat unit per cub. foot).
| Constituents. | Volume per cent. |
Weight per cent. |
Heat-value per Cubic Metre Calories. |
Heat-value per Quantity contained in 1 Cub. Met. |
Heat-value per cent. of Total. |
| Hydrogen, H2 | 47 | 7.4 | 2,582 | 1213 | 22.8 |
| Methane, CH4 | 34 | 42.8 | 8,524 | 2898 | 54.5 |
| Carbon monoxide, CO | 9 | 19.9 | 3,043 | 273 | 5.1 |
| Benzene vapour, C6H6 | 1.2 | 7.4 | 33,815 | 405 | 7.7 |
| Ethylene, C2H4 | 3.8 | 8.4 | 13,960 | 530 | 9.9 |
| Carbon dioxide, CO2 | 2.5 | 8.6 | .. | .. | .. |
| Nitrogen, N2 | 2.5 | 5.5 | .. | .. | .. |
| Total | 100.0 | 100.0 | .. | 5319 | 100.0 |
One cubic metre of such gas weighs 568 grammes. Rich gas, or gas made by the destructive distillation of certain bituminous schists, of oil, &c., contains much more of the heavy hydrocarbons, and its heat-value is therefore much higher than the above. The carburetted water gas, very generally made in America, and sometimes employed in England for mixing with coal gas, is of varying composition; its heat-value is generally rather less than that of coal gas (see below).
IV. Combustible Gases produced by the Partial Combustion of Coal, &c.—These form by far the most important kind of gaseous fuel. When coal is submitted to destructive distillation to produce the illuminating gas described in the preceding paragraph, only a comparatively small proportion of the heating value of the coal (say, a sixth or at most a fifth part) is obtained in the shape of gaseous fuel, by far the greater proportion remaining behind in the shape of coke.
An entirely different class of gaseous fuels comprises those produced by the incomplete combustion of the total carbon contained in the raw material, where the result is a mixture of gases which, being capable of combining with more oxygen, can be burnt and employed for heating purposes. Apart from some descriptions of waste gases belonging to this class (of which the most notable are those from blast-furnaces), we must distinguish two ways of producing such gaseous fuels entirely different in principle, though sometimes combined in one operation. The incomplete combustion of carbon may be brought about by means of atmospheric oxygen, by means of water, or by a simultaneous combination of these two actions. In the first case the chemical reaction is
C + O = CO
the nitrogen accompanying the oxygen in the atmospheric air necessarily remains mixed with carbon monoxide, and the resulting gases, which always contain some carbon dioxide, some products of the destructive distillation of the coal, &c., are known as producer gas or Siemens gas. In the second case the chemical reaction is mainly
C + H2O = CO + H2
that is to say, the carbon is converted into monoxide and the hydrogen is set free. As both of these substances can combine with oxygen, and as there is no atmospheric nitrogen to deal with, the resulting gas (water gas) is, apart from a few impurities, entirely combustible. Another kind of water gas is formed by the reaction
C + 2H2O = CO2 + 2H2
but this reaction, which converts all the carbon into the incombustible form of CO2, is considered as an unwelcome, although never entirely avoidable, concomitant of (b).
The reaction by which water gas is produced being endothermic (as we shall see), this gas cannot be obtained except by introducing the balance of energy in another manner. This might be done by heating the apparatus from without, but as this method would be uneconomical, the process is carried out by alternating the endothermic production of water gas with the exothermic combustion of carbon by atmospheric air. Pure water gas is not, therefore, made by a continuous process, but alternates with the production of other gases, combustible or not. But instead of constantly interrupting the process in this way, a continuous operation may be secured by simultaneously carrying on both the reactions (a) and (b) in such proportions that the heat generated by (a) at least equals the heat absorbed by (b). For this purpose the apparatus is fed at the same time with atmospheric air and with a certain quantity of steam, preferably in a superheated state. Gaseous mixtures of this kind have been made, more or less intentionally, for a long time past. One of the best known of them, intended less for the purpose of serving as ordinary fuel than for that of driving machinery, is the Dowson gas.
An advantage common to all kinds of gaseous fuel, which indeed forms the principal reason why it is intentionally produced from solid fuel, in spite of inevitable losses in the course of the operation, is the following. The combustion of solid fuel (coal, &c.) cannot be carried on with the theoretically necessary quantity of atmospheric air, but requires a considerable excess of the latter, at least 50%, sometimes 100% and more. This is best seen from the analyses of smoke gases. If all the oxygen of the air were converted into CO2 and H2O, the amount of CO2 in the smoke gases should be in the case of pure carbon nearly 21 volumes %, as carbon dioxide occupies the same volume as oxygen; while ordinary coal, where the hydrogen takes up a certain quantity of oxygen as well, should show about 18.5% CO2. But the best smoke gases of steam boilers show only 12 or 13%, much more frequently only 10% CO2, and gases from reverberatory furnaces often show less than 5%. This means that the volume of the smoke gases escaping into the air is from 1½ to 2 times (in the case of high-temperature operations often 4 times) greater than the theoretical minimum; and as these gases always carry off a considerable quantity of heat, the loss of heat is all the greater the less complete is the utilization of the oxygen and the higher the temperature of the operation. This explains why, in the case of the best-constructed steam-boiler fires provided with heat economizers, where the smoke gases are deprived of most of their heat, the proportion of the heat value of the fuel actually utilized may rise to 70 or even 75%, while in some metallurgical operations, in glass-making and similar cases, it may be below 5%.
One way of overcoming this difficulty to a certain extent is to reduce the solid fuel to a very fine powder, which can be intimately mixed with the air so that the consumption of the latter is only very slightly in excess of the theoretical quantity; but this process, which has been only recently introduced on a somewhat extended scale, involves much additional expense and trouble, and cannot as yet be considered a real success. Generally, too, it is far less easily applied than gaseous fuel. The latter can be readily and intimately mixed with the exact quantity of air that is required and distributed in any suitable way, and much of the waste heat can be utilized for a preliminary heating of the air and the gas to be burned by means of “recuperators.”
We shall now describe the principal classes of gaseous fuel, produced by the partial combustion of coal.
A. Producer Gas, Siemens Gas.—As we have seen above, this gas is made by the incomplete combustion of fuel. The materials generally employed for its production are anthracite, coke or other fuels which are not liable to cake during the operation, and thus stop the draught or otherwise disturb the process, but by special measures also bituminous coal, lignite, peat and other fuel may be utilized for gas producers. The fuel is arranged in a deep layer, generally from 4 ft. up to 10 ft., and the air is introduced from below, either by natural draught or by means of a blast, and either by a grate or only by a slit in the wall of the “gas producer.” Even if the primary action taking place at the entrance of the air consisted in the complete combustion of the carbon to dioxide, CO2, the latter, in rising through the high column of incandescent fuel, must be reduced to monoxide: CO2 + C = 2CO. But as the temperature in the producer rises rather high, and as in ordinary circumstances the action of oxygen on carbon above 1000° C. consists almost entirely in the direct formation of CO, we may regard this compound as primarily formed in the hotter parts of the gas-producer. It is true that ordinary producer gas always contains more or less CO2, but this may be formed higher up by air entering through leakages in the apparatus. If we ignore the hydrogen contained in the fuel, the theoretical composition of producer gas would be 33.3% CO and 66.7% N, both by volume and weight. Its weight per cubic metre is 1.251 grammes, and its heat value 1013 calories per cubic metre, or less than one-fifth of the heat-value of coal gas. Practically, however, producer gas contains a small percentage of gases, increasing its heat-value, like hydrogen, methane, &c., but on the other hand it is never free from carbon dioxide to the extent of from 2 to 8%. Its heat-value may therefore range between 800 and 1100 calories per cubic metre. Even when taking as the basis of our calculation a theoretical gas of 33.3% CO, we find that there is a great loss of heat-value in the manufacture of this gas. Thermochemistry teaches us that the reaction C + O develops 29.5% of the heat produced by the complete oxidation of C to CO2, thus leaving only 70.5% for the stage CO + O = CO2. If, therefore, the gas given off in the producer is allowed to cool down to ordinary temperature, nearly 30% of the heat-value of the coal is lost by radiation. If, however, the gas producer is built in close proximity to the place where the combustion takes place, so that the gas does not lose very much of its heat, the loss is correspondingly less. Even then there is no reason why this mode of burning the fuel, i.e. first with “primary air” in the producer (C + O = CO), then with “secondary air” in the furnace (CO + O =CO2), should be preferred to the direct complete burning of the fuel on a grate, unless the above-mentioned advantage is secured, viz. reduction of the smoke gases to a minimum by confining the supply of air as nearly as possible to that required for the formation of CO2, which is only possible by producing an intimate mixture of the producer gas with the secondary air. The advantage in question is not very great where the heat of the smoke gases can be very fully utilized, e.g. in well-constructed steam boilers, salt-pans and the like, and as a matter of fact gas producers have not found much use in such cases. But a very great advantage is attained in high-temperature operations, where the smoke gases escape very hot, and where it is on that account all-important to confine their quantity to a minimum.
It is precisely in these cases that another requirement frequently comes in, viz. the production at a given point of a higher temperature than is easily attained by ordinary fires. Gas-firing lends itself very well to this end, as it is easily combined with a preliminary heating up of the air, and even of the gas itself, by means of “recuperators.” The original and best-known form of these, due to Siemens Brothers, consists of two brick chambers filled with loosely stacked fire-bricks in such manner that any gases passed through the chambers must seek their way through the interstices left between the bricks, by which means a thorough interchange of temperature takes place. The smoke gases, instead of escaping directly into the atmosphere, are made to pass through one of these chambers, giving up part of their heat to the brickwork. After a certain time the draught is changed by means of valves, the smoke gases are passed through another chamber, and the cold air intended to feed the combustion is made to pass through the first chamber, where it takes up heat from the white-hot bricks, and is thus heated up to a bright red heat until the chamber is cooled down too far, when the draughts are again reversed. Sometimes the producer gas itself is heated up in this manner (especially when it has been cooled down by travelling a long distance); in that case four recuperator chambers must be provided instead of two. Another class of recuperators is not founded on the alternating system, but acts continuously; the smoke gases travel always in the same direction in flues contiguous to other flues or pipes in which the air flows in the opposite direction, an interchange of heat taking place through the walls of the flues or pipes. Here the surface of contact must be made very large if a good effect is to be produced. In both cases not merely is a saving effected of all the calories which are abstracted by the cold air from the recuperator, but as less fuel has to be burned to get a given effect, the quantity of smoke gas is reduced. For details and other producer gases, see Gas, II. For Fuel and Power.
Gas-firing in the manner just described can be brought about by very simple means, viz. by lowering the fire-grate of an ordinary fire-place to at least 4 ft. below the fire-bridge, and by introducing the air partly below the grate and partly behind the fire-place, at or near the point where the greatest heat is required. Usually, however, more elaborate apparatus is employed, some of which we shall describe below. Gas-firing has now become universal in some of the most important industries and nearly so in others. The present extension of steel-making and other branches of metallurgy is intimately connected with this system, as is the modern method of glass-making, of heating coal gas retorts and so forth.
The composition of producer gas differs considerably, principally according to the material from which it is made. Analyses of ordinary producer gas (not such as falls under the heading of “semi-water gas,” see sub C) by volume show 22 to 33% CO, 1 to 7% CO2, 0.5 to 2% H2, 0.5 to 3% hydrocarbons, and 64 to 68% N2.
B. Water Gas.—The reaction of steam on highly heated carbonaceous matter was first observed by Felice Fontana in 1780. This was four years before Henry Cavendish isolated hydrogen from water, and thirteen years before William Murdoch made illuminating gas by the distillation of coal, so that it was no wonder that Fontana’s laboratory work was soon forgotten. Nor had the use of carburetted water gas, as introduced by Donovan in 1830 for illuminating purposes, more than a very short life. More important is the fact that during nine years the illumination of the town of Narbonne was carried on by incandescent platinum wire, heated by water gas, where also internally heated generators were for the first time regularly employed. The Narbonne process was abandoned in 1865, and for some time no real progress was made in this field in Europe. But in America, T.S.C. Lowe, Strong, Tessié du Motay and others took up the matter, the first permanent success being obtained by the introduction (1873) of Lowe’s system at Phoenixville, Pa. In the United States the abundance of anthracite, as well as of petroleum naphtha, adapted for carburetting the gas, secures a great commercial advantage to this kind of illuminant over coal gas, so that now three-fourths of all American gas-works employ carburetted water gas. In Europe the progress of this industry was naturally much less rapid, but here also since 1882, when the apparatus of Lowe and Dwight was introduced in the town of Essen, great improvements have been worked out, principally by E. Blass, and by these improvements water gas obtained a firm footing also for certain heating purposes. The American process for making carburetted water gas, as an auxiliary to ordinary coal gas, was first introduced by the London Gas Light and Coke Company on a large scale in 1890.
Water gas in its original state is called “blue gas,” because it burns with a blue, non-luminous flame, which produces a very high temperature. According to the equation C + H2O = CO + H2, this gas consists theoretically of equal volumes of carbon monoxide and hydrogen. We shall presently see why it is impossible to avoid the presence of a little carbon dioxide and other gases, but we shall for the moment treat of water gas as if it were composed according to the above equation. The reaction C + H2O = CO + H2 is endothermic, that is, its thermal value is negative. One gram-molecule of carbon produces 97 great calories (1 great calorie or kilogram-calorie = 1000 gram-calories) when burning to CO2, and this is of course the maximum effect obtainable from this source. If the same gram-molecule of carbon is used for making water gas, that is, CO + H2, the heat produced by the combustion of the product is 68.4 + 57.6 = 126 great calories, an apparent surplus of 29 calories, which cannot be got out of nothing. This is made evident by another consideration. In the above reaction C is not burned to CO2, but to CO, a reaction which produces 28.6 calories per gram-molecule. But as the oxygen is furnished from water, which must first be decomposed by the expenditure of energy, we must introduce this amount, 68.5 calories in the case of liquid water, or 57.6 calories in the case of steam, as a negative quantity, and the difference, viz. + 28.6 − 57.6 = 29 great calories, represents the amount of heat to be expended from another source in order to bring about the reaction of one gram-molecule of carbon on one gram-molecule of H2O in the shape of steam. This explains why steam directed upon incandescent coal will produce water gas only for a very short time: even a large mass of coal will quickly be cooled down so much that at first a gas of different composition is formed and soon the process will cease altogether. We can avoid this result by carrying on the process in a retort heated from without by an ordinary coal fire, and all the early water gas apparatus was constructed in this way; but such a method is very uneconomical, and was long ago replaced by a process first patented by J. and T.N. Kirkham in 1854, and very much improved by successive inventors. This process consists in conducting the operation in an upright brick shaft, charged with anthracite, coke or other suitable fuel. This shaft resembles an ordinary gas producer, but it differs in being worked, not in a continuous manner, which, as shown above, would be impossible, but by alternately blowing air and steam through the coal for periods of a few minutes each. During the first phase, when carbon is burned by atmospheric oxygen, and thereby heat is produced, this heat, or rather that part of it which is not carried away by radiation and by the products of combustion on leaving the apparatus, is employed in raising the temperature of the remaining mass of fuel, and is thus available for the second phase, in which the reaction (b) C + H2O = CO + H2 goes on with the abstraction of a corresponding amount of heat from the incandescent fuel, so that the latter rapidly cools down, and the process must be reversed by blowing in air and so forth. The formation of exactly equal volumes of carbon monoxide and hydrogen goes on only at temperatures over 1200° C., that is, for a very few minutes. Even at 1100° C. a little CO2 can be proved to exist in the gas, and at 900° its proportion becomes too high to allow the process to go on. About 650° C. the CO has fallen to a minimum, and the reaction is now essentially (c) C + 2H2O = CO2 + 2H2; soon after the temperature of the mass will have fallen to such a low point that the steam passes through it without any perceptible action. The gas produced by reaction (c) contains only two-thirds of combustible matter, and is on that account less valuable than proper water gas formed by reaction (b); moreover, it requires the generation of twice the amount of steam, and its presence is all the less desirable since it must soon lead to a total cessation of the process. In ordinary circumstances it is evident that the more steam is blown in during a unit of time, the sooner reaction (c) will set in; on the other hand, the more heat has been accumulated in the producer the longer can the blowing-in of steam be continued.
The process of making water gas consequently comprises two alternating operations, viz. first “blowing-up” by means of a current of air, by which the heat of the mass of fuel is raised to about 1200° C.; and, secondly “steaming,” by injecting a current of (preferably superheated) steam until the temperature of the fuel had fallen to about 900° C., and too much carbon dioxide appears in the product. During the steaming the gas is carried off by a special conduit into a scrubber, where the dust mechanically carried away in the current is washed out, and the gas is at the same time cooled down nearly to the ordinary temperature. It is generally stored in a gas-holder, from which it is conducted away as required. It is never quite free from nitrogen, as the producer at the beginning of steaming contains much of this gas, together with CO or CO2. The proportion of hydrogen may exceed 50%, in consequence of reaction (c) setting in at the close of the steaming. Ordinary “blue” water gas, if, as usual, made from coke or anthracite, contains 48-52% H2, 40-41% CO, 1-5% CO2, 4-5% N2, and traces of hydrocarbons, especially methane. If made from bituminous coal, it contains more of the latter. If “carburetted” (a process which increases its volume 50% and more) by the vapours from superheated petroleum naphtha, the proportion of CO ranges about 25%, with about as much methane, and from 10 to 15% of “illuminants” (heavy hydrocarbons). The latter, of course, greatly enhance the fuel-value of the gas. Pure water gas would possess the following fuel-value per cubic metre:
| 0.5 | cub. met. | H2 | = 1291 | calories |
| 0.5 | ” ” | CO | = 1522 | ” |
| 2813 | ” | |||
Ordinary “blue” water gas has a fuel-value of at least 2500 calories. Carburetted water gas, which varies very much in its percentage of hydrocarbons, sometimes reaches nearly the heat-value of coal gas, but such gas is only in exceptional cases used for heating purposes.
We must now turn to the “blowing-up” stage of the process. Until recently it was assumed that during this stage the combustion of carbon cannot be carried on beyond the formation of carbon monoxide, for as the gas-producer must necessarily contain a deep layer of fuel (generally about 6 to 10 ft.), any CO2 formed at first would be reduced to CO; and it was further assumed that hardly any CO2 would be formed from the outset, as the temperature of the apparatus is too high for this reaction to take place. But as the combustion of C to CO produces only about 30% of the heat produced when C is burned into CO2, the quantity of fuel consumed for “blowing-up” is very large, and in fact considerably exceeds that consumed in “steaming.” There is, of course, a further loss by radiation and minor sources, and the result is that 1 kilogram of carbon yields only about 1.2 cub. met. of water gas. Each period of blowing-up generally occupies from 8 to 12 minutes, that of steaming only 4 or 5 minutes. This low yield of water gas until quite recently appeared to be unavoidable, and the only question seemed to be whether and to what extent the gas formed during blowing-up, which is in fact identical with ordinary producer gas (Siemens gas), could be utilized. In America, where the water gas is mostly employed for illuminating purposes, at least part of the blowing-up gas is utilized for heating the apparatus in which the naphtha is volatilized and the vapours are “fixed” by superheating. This process, however, never utilizes anything like the whole of the blowing-up gas, nor can this be effected by raising and superheating the steam necessary for the second operation; indeed, the employment of this gas for raising steam is not very easy, owing to the irregularities of and constant interruptions in the supply. In some systems the gas made during the blowing-up stage is passed through chambers, loosely filled with bricks, like Siemens recuperators, where it is burned by “secondary” air: the heat thus imparted to the brickwork is utilized by passing through the recuperator, and thus superheating, the steam required for the next steaming operation. In many cases, principally where no carburetting is practised, the blowing-up gas is simply burned at the mouth of the producer, and is thus altogether lost; and in no case can it be utilized without great waste. A very important improvement in this respect was effected by C. Dellwik and E. Fleischer. They found that the view that it is unavoidable to burn the carbon to monoxide during the blowing-up holds good only for the pressure of blast formerly applied. This did not much exceed that which is required for overcoming the frictional resistance within the producer. If, however, the pressure is considerably increased, and the height of the column of fuel reduced, both of these conditions being strictly regulated in accordance with the result desired, it is easy to attain a combustion of the carbon to dioxide, with only traces of monoxide, in spite of the high temperature. Evidently the excess of oxygen coming into contact with each particle of carbon in a given unit of time produces other conditions of chemical equilibrium than those existing at lower pressures. At any rate, experience has shown that by this process, in which the full heat-value of carbon is utilized during the blowing-up stage, the time of heating-up can be reduced from 10 to 1½ or 2 minutes, and the steaming can be prolonged from 4 or 5 to 8 or 10 minutes, with the result that twice the quantity of water gas is obtained, viz. upwards of 2 cub. metres from 1 kilogram of carbon.
The application of water gas as a fuel mainly depends upon the high temperatures which it is possible to attain by its aid, and these are principally due to the circumstance that it forms a much smaller flame than coal gas, not to speak of Siemens gas, which contains at most 33% of combustible matter against 90% or more in water gas. The latter circumstance also allows the gas to be conducted and distributed in pipes of moderate dimensions. Its application, apart from its use as an illuminant (with which we are not concerned here), was formerly retarded by its high cost in comparison with Siemens gas and other sources of heat, but as this state of affairs has been changed by the modern improvements, its use is rapidly extending, especially for metallurgical purposes.
C. Mixed Gas (Semi-Water Gas).—This class is sometimes called Dowson gas, irrespective of its method of production, although it was made and extensively used a long time before J.E. Dowson constructed his apparatus for generating such a gas principally for driving gas-engines. By a combination of the processes for generating Siemens gas and water gas, it is produced by injecting into a gas-producer at the same time a certain quantity of air and a corresponding quantity of steam, the latter never exceeding the amount which can be decomposed by the heat-absorbing reaction, C + H2O = CO + H2, at the expense of the heat generated by the action of the air in the reaction C + O = CO. Such gas used to be frequently obtained in an accidental way by introducing liquid water or steam into an ordinary gas-producer for the purpose of facilitating its working by avoiding an excessive temperature, such as might cause the rapid destruction of the brickwork and the fusion of the ashes of the fuel into troublesome cakes. It was soon found that by proceeding in this way a certain advantage could be gained in regard to the consumption of fuel, as the heat abstracted by the steam from the brickwork and the fuel itself was usefully employed for decomposing water, its energy thus reappearing in the shape of a combustible gas. It is hardly necessary to mention explicitly that the total heat obtained by any such process from a given quantity of carbon (or hydrogen) can in no case exceed that which is generated by direct combustion; some inventors, however, whether inadvertently or intentionally, have actually represented this to be possible, in manifest violation of the law of the conservation of energy.
Roughly speaking, this gas may be said to be produced by the combination of the reactions, described sub A and B, to the joint reaction: 2C + O + H2O = 2CO + H2. The decomposition of H2O (applied in the shape of steam) absorbs 57.6 gram calories, the formation of 2CO produces 59 gram calories; hence there is a small positive excess of 1.4 calories at disposal. This in reality would not be sufficient to cover the loss by radiation, &c.; hence rather more free oxygen (i.e. atmospheric air) must be employed than is represented by the above equation. All this free oxygen is, of course, accompanied by nearly four times its volume of nitrogen.
The mixed gas thus obtained differs very much in composition, but is always much richer in hydrogen (of which it contains sometimes as much as 20%) and poorer in carbon monoxide (sometimes down to 20%) than Siemens gas; generally it contains more of CO2 than the latter. The proportion of nitrogen is always less, about 50%. It is therefore a more concentrated fuel than Siemens gas, and better adapted to the driving of gas-engines. It scarcely costs more to make than ordinary Siemens gas, except where the steam is generated and superheated in special apparatus, as is done in the Dowson producer, which, on the other hand, yields a correspondingly better gas. As is natural, its properties are some way between those of Siemens gas and of water gas; but they approach more nearly the former, both as to costs and as to fuel-value, and also as to the temperatures reached in combustion. This is easily understood if we consider that gas of just the same description can be obtained by mixing one volume of real water gas with the four volumes of Siemens gas made during the blowing-up stage—an operation which is certainly too expensive for practical use.
A modification of this gas is the Mond gas, which is made, according to Mond’s patent, by means of such an excess of steam that most of the nitrogen of the coke is converted into ammonia (Grouven’s reaction). Of course much of this steam passes on undecomposed, and the quantity of the gas is greatly increased by the reaction C + 2H2O = CO2 + 2H2; hence the fuel-value of this gas is less than that of semi-water gas made in other ways. Against this loss must be set the gain of ammonia which is recovered by means of an arrangement of coolers and scrubbers, and, except at very low prices of ammonia, the profit thus made is probably more than sufficient to cover the extra cost. But as the process requires very large and expensive plant, and its profits would vanish in the case of the value of ammonia becoming much lower (a result which would very probably follow if it were somewhat generally introduced), it cannot be expected to supplant the other descriptions of gaseous fuel to more than a limited extent.
Semi-water gas is especially adapted for the purpose of driving gas-engines on the explosive principle (gas-motors). Ordinary producer-gas is too poor for this purpose in respect of heating power; moreover, owing to the prevalence of carbon monoxide, it does not light quickly enough. These defects are sufficiently overcome in semi-water gas by the larger proportion of hydrogen contained in it. For the purpose in question the gas should be purified from tar and ashes, and should also be cooled down before entering the gas-engine. The Dowson apparatus and others are constructed on this principle.
Air Gas.—By forcing air over or through volatile inflammable liquids a gaseous mixture can be obtained which burns with a bright flame and which can be used for illumination. Its employment for heating purposes is quite exceptional, e.g. in chemical laboratories, and we abstain, therefore, from describing any of the numerous appliances, some of them bearing very fanciful names, which have been devised for its manufacture.


