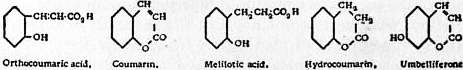
Coumarin, C9H6O2, a substance which occurs naturally in sweet woodruff (Asperula odorata), in the tonka bean and in yellow melilot (Melilotus officinalis). It can be obtained from the tonka bean by extraction with alcohol. It is prepared artificially by heating aceto-ortho-coumaric acid (which is formed from sodium salicyl aldehyde) or from the action of acetic anhydride and sodium acetate on salicyl aldehyde (Sir W. H. Perkin, Berichte, 1875, 8, p. 1599). It can also be prepared by heating a mixture of phenol and malic acid with sulphuric acid, or by passing bromine vapour at 107° C. over the anhydride of melilotic acid. It forms rhombic crystals (from ether) melting at 67° C. and boiling at 290° C., which are readily soluble in alcohol, and moderately soluble in hot water. It is applied in perfumery for the preparation of the Asperula essence. On boiling with concentrated caustic potash it yields the potassium salt of coumaric acid, whilst when fused with potash it is completely decomposed into salicylic and acetic acids. Sodium amalgam reduces it, in aqueous solution, to melilotic acid. It forms addition products with bromine and hydrobromic acid. By the action of phosphorus pentasulphide it is converted into thiocoumarin, which melts at 101° C.; and in alcoholic solution, on the addition of hydroxylamine hydrochloride and soda, it yields coumarin oxime.
Ortho-coumaric acid (o-oxycinnamic acid) is obtained from coumarin as shown above, or by boiling coumarin for some time with sodium ethylate. It melts at 208° C. and is easily soluble in hot water and in alcohol. It cannot be converted into coumarin by heating alone, but it is readily transformed on heating with acetic anhydride or acetyl chloride. By the action of sodium amalgam it is readily converted into melilotic acid, which melts at 81° C., and on distillation furnishes its lactone, hydrocoumarin, melting at 25° C. For the relations of coumaric and coumarinic acid see Annalen, 254, p. 181. The homologues of coumarin may be obtained by the action of sulphuric acid on phenol and the higher fatty acids (propionic, butyric and isovaleric anhydrides), substitution taking place at the carbon atom in the α position to the -CO- group, whilst by the condensation of acetoacetic ester and phenols with sulphuric acid the β substituted coumarins are obtained.
Umbelliferone or 4-oxycoumarin, occurs in the bark of Daphne mezereum and may be obtained by distilling such resins as galbanum or asafoetida. It may be synthesized from resorcin and malic anhydride or from β resorcyl aldehyde, acetic anhydride and sodium acetate. Daphnetin and Aesculetin are dioxycoumarins.
The structural formulae of coumarin and the related substances are:
 |


