Atom (Gr. ἄτομος, indivisible, from ἀ- privative, and τέμνειν, to cut), the term given in physical science to the ultimate indivisible particle of matter, and so by analogy to something minutely small in size. If we examine such a substance as sugar we find that it can be broken up into fine grains, and these again into finer, the finest particles still appearing to be of the same nature as sugar. The same is true in the case of a liquid such as water; it can be divided into drops and these again into smaller drops, or into the finest spray the particles of which are too small to be detected by our unaided vision. In fact, so far as the direct evidence of our senses tells us, matter appears to be indefinitely divisible. Moreover, small particles do not seem to exist in the water until it is broken up; so far as we can see, the material of the water is continuous not granular. This conception of matter, as infinitely divisible and continuous, was taught by Anaxagoras more than four centuries before the Christian era, and in the philosophy of Aristotle the same ideas are found. Theories of matter. But some phenomena are difficult to reconcile with this view; for example, a cubic foot of air can be compressed into less than one five-hundredth of a cubic foot, or, if allowed to expand, the air originally occupying the cubic foot can be made to fill, apparently uniformly, a space of a million cubic feet or more. This enormous capacity for expansion and contraction is astonishing if we believe matter to be continuous, but if we imagine air to be made up of little particles separated by relatively large empty spaces the changes in volume are more easily conceivable. Moreover, if we attribute such a structure to gases, we are led to attribute it to liquids and to solids also, since gases can be liquefied without any abrupt change, and many substances usually solid can be converted into gases by heating them. This conception of the grained structure of matter is very ancient; traces of it are to be found in Indian philosophy, perhaps twelve centuries before the Christian era, and the Greek philosophers Democritus and Epicurus, in the 3rd and 4th centuries B.C., taught it very definitely. Their view was that “matter is not indefinitely divisible, but that all substances are formed of indivisible particles or atoms which are eternal and unchangeable, that the atoms are separated from one another by void, and that these atoms, by their combinations, form the matter we are conscious of.” The Roman poet Lucretius (De Rerum Natura) was an eloquent exponent of this theory, but throughout the middle ages, indeed until the 17th century, it was eclipsed by the prestige of Aristotle. In the time, however, of Boyle1 and Newton, we again find an atomic theory of matter; Newton2 regarded a gas as consisting of small separate particles which repelled one another, the tendency of a gas to expand being attributed to the supposed repulsion between the particles.
Let us consider some common phenomena in the light of these rival theories as to the nature of matter. When a few lumps of sugar are added to a glass of water and stirred, the sugar soon disappears and we are left with a uniform liquid resembling water, except that it is sweet. What has become of the sugar? Does it still exist? The atomist would say, “Yes, it is broken up into its atoms, and these are distributed throughout the spaces between the particles of water.” The rival philosopher, who believes water to be continuous and without spaces between its particles, has a greater difficulty in accounting for the disappearance of the sugar; he would probably say that the sugar, and the water also, had ceased to exist, and that a new continuous substance had been formed from them, but he could offer no picture of how this change had taken place. Or consider a well-marked case of what we are in the habit of calling chemical combination. If 127 parts of iodine, which is an almost black solid, and 100 parts of mercury, which is a white liquid metal, be intimately mixed by rubbing them together in a mortar, the two substances wholly disappear, and we obtain instead a brilliant red powder quite unlike the iodine or the mercury; almost the only property that is unchanged is the weight. The question again arises, what has become of the original substances? The atomist has an easy answer; he says that the new body is made up by the juxtaposition of the atoms of iodine and mercury, which still exist in the red powder. His opponent would be disposed to say that the iodine and the mercury ceased to exist when the red powder was formed, that they were components but not constituents of it. The fact that the two components can be recovered from the compound by destroying it does not decide the question. It is remarkable that pure chemistry, even to-day, has no very conclusive arguments for the settlement of this controversy; but the sister science of physics is steadily accumulating evidence in favour of the atomic conception.
 |
||
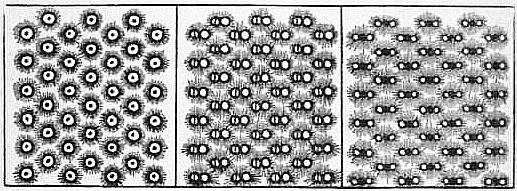
| From Dalton’s New System of Chemical Philosophy. | ||
| Hydrogen Gas. | Nitrous Gas. | Carbonic Acid Gas. |
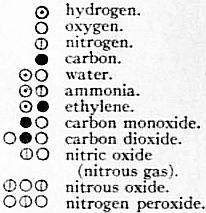 |
Until the time of John Dalton, the atomic conception remained purely qualitative, and until then it does not appear to have advanced chemistry or to have found further confirmation in the facts of chemistry. Dalton (1803) gave the atomic theory a Dalton. quantitative form, and showed that, by means of it, a vast number of the facts of chemistry could be predicted or explained. In fact, he did so much to make the atomic theory of matter probable that he is popularly regarded as its originator. Dalton lived in a period marked by great advances in experimental chemistry. Rather before the commencement of the 19th century the work of Lavoisier had rendered it very probable that chemical changes are not accompanied by any change in weight, and this principle of the conservation of matter was becoming universally accepted; chemists were also acquiring considerable skill in chemical analysis, that is, in the determination of the nature and relative amounts of the elements contained in compounds. But Sir H.E. Roscoe and A. Harden, New View of the Atomic Theory (1896), have shown, from a study of Dalton’s manuscript notes, that we do not owe his atomic theory to such experiments. If their view is correct, the theory appears to be a remarkable example of deductive reasoning. Dalton, who was a mathematical physicist even more than a chemist, had given much thought to the study of gases. Following Newton, he believed a gas to be made up of particles or atoms, separated from one another by considerable spaces. Certain difficulties that he met with in his speculations led him to the conclusion that the particles of any one kind of gas, though all of them alike, must differ from those of another gas both in size and weight. He thus arrived at the conception of a definite atomic weight peculiar to the particles of each gas, and he thought that he could determine these atomic weights, in terms of one of them, by means of the quantitative analysis of compounds. The conclusion that each element had a definite atomic weight, peculiar to it, was the new idea that made his speculations fruitful, because it allowed of quantitative deduction and verification. He drew simple diagrams, three of which, taken from Dalton’s New System of Chemical Philosophy, part ii. (1810), are reproduced here, in which gases are represented as composed of atoms. Knowing that the gas which he called “nitrous gas” was composed of oxygen and nitrogen, and believing it to be the simplest compound of these two elements, he naturally represented its atom as formed of an atom of oxygen and an atom of nitrogen in juxtaposition. When two elements form more than one compound, as is the case with oxygen and carbon, he assigned to the compound which he thought the more complex an atom made up of two atoms of the one element and one atom of the other; the diagram for carbonic acid illustrates this, and an extension of the same plan enabled him to represent any compound, however complex its structure. The table here given contains some of Dalton’s diagrams of atoms. They are not all considered to be correct at the present time; for example, we now think that the ultimate particle of water is made up of two atoms of hydrogen and one of oxygen, and that that of ammonia contains three atoms of hydrogen to one of nitrogen. But these differences between Dalton’s views and our present ones do not impair the accuracy of the arguments which follow. The diagrams show that Dalton formed a very definite conception of the nature of chemical combination; it was the union of a small number of atoms of one kind with a small number of another kind to form a compound atom, or as we now say a “molecule,” this identical process being repeated millions of times to form a perceptible amount of a compound. The conceptions of “element,” “compound” and “mixture” became more precise than they had been hitherto; in an element all the atoms are alike, in a compound all the molecules are alike, in a mixture there are different kinds of molecules. If we accept the hypothesis that each kind of atom has a specific and invariable weight, we can, with the aid of the above theory, make most important inferences concerning the proportions by weight in which substances combine to form compounds. These inferences are often summarized as the laws of constant, multiple and reciprocal proportions.
The law of constant proportions asserts that when two elements unite to form a compound the weights that combine are in an invariable ratio, a ratio that is characteristic of that compound. Thus if Dalton’s diagram for the molecule, Law of constant proportions. or compound atom, of water be correct, it follows that in all samples of water the total number of the hydrogen atoms is equal to that of the oxygen atoms; consequently, the ratio of the weight of oxygen to that of hydrogen in water is the same as the ratio of the weights of an oxygen and a hydrogen atom, and this is invariable. Different samples of water cannot therefore differ ever so little in percentage composition, and the same must be true for every compound as distinguished from a mixture. Apart from the atomic theory there is no obvious reason why this should be so. We give the name bread to a substance containing variable proportions of flour and water. Similarly the substance we call wine is undeniably variable in composition. Why should not the substance we call water also vary more or less? The Aristotelian would find no difficulty in such a variability; it is only the disciple of Dalton to whom it seems impossible. It is evident that we have in this law a definite prediction that can be tested by experiment.
The law of multiple proportions asserts that if two elements form more than one compound, then the weights of the one element which are found combined with unit weight of the other in the different compounds, must be in the ratio of two or more whole numbers. If we compare Dalton’s Law of multiple proportions. diagrams of the two oxides of carbon or of the three oxides of nitrogen that are given in the preceding table, we at once see the necessity of this law; for the more complex molecule has to be formed from the simpler one by the addition of one or more whole atoms. In the oxides of carbon the same weight of carbon must be combined with weights of oxygen that are as 1 : 2, and in the oxides of nitrogen a fixed weight of nitrogen must be in union with weights of oxygen that are as 1 : 2 : ½, which are the same ratios as 2 : 4 : 1. This law has been abundantly verified by experiment; for example, five oxides of nitrogen are known, and independent analyses show that, if we consider the same weight of nitrogen in every case, the weights of oxygen combined with it are to one another as 1 : 2 : 3 : 4 : 5. The discovery of this law is due to Dalton; it is a direct deduction from his atomic theory. Here again, apart from this theory, there is no obvious reason why the composition of different substances should be related in so simple a way. As Dalton said, “The doctrine of definite proportions appears mysterious unless we adopt the atomic hypothesis.” “It appears like the mystical ratios of Kepler which Newton so happily elucidated.” The chemists of Dalton’s time were not unanimous in accepting these laws; indeed C.L. Berthollet (Essai de statique chimique, 1803) expressly controverted them. He maintained that, under varying conditions, two substances could combine in an indefinitely large number of different ratios, that there could in fact be a continuous variation in the combining ratio. This view is clearly inconsistent with the atomic theory, which requires that when the combining ratio of two substances changes it should do so, per saltum, to quite another value.
The law of reciprocal proportions, or, as it might well be named, the law of equivalence, cannot be adequately enunciated in a few words. The following gives a partial statement of it. Law of reciprocal proportions. If we know the weights a and b of two elements that are found in union with unit weight of a third element, then we can predict the composition of the compounds which the first two elements can form with each other; either the weights a and b will combine exactly, or if not, these weights must be multiplied by integers to obtain the composition of a compound. To see how this law follows from Dalton’s theory let us consider his diagrams for the molecules of water, ethylene and the oxides of carbon. In water and in ethylene experiment shows that 8 parts by weight of oxygen and 6 parts of carbon, respectively, are in union with one part of hydrogen; also, if the diagrams are correct, these numbers must be in the ratio of the atomic weights of oxygen and carbon. We can therefore predict that all oxides of carbon will have compositions represented by the ratio of 8m parts of oxygen to 6n parts of carbon, where m and n are whole numbers. This prediction is verified by the result of analysis. Similarly, if we know by experiment the composition of water and of ammonia, we can predict the probable composition of the oxides of nitrogen. Experiment shows that, in water and ammonia, we have, respectively, 8 parts of oxygen and 4.67 parts of nitrogen in union with one part of hydrogen; we can therefore infer that the oxides of nitrogen will all have the composition of 8m parts of oxygen to 4.67n parts of nitrogen. Experiment alone can tell us the values of m and n; all that the theory tells us is that they are whole numbers. In this particular case, n turns out to be 3, and m has in succession the values 1, 2, 3, 4, 5.
It is evident that these laws all follow from the idea that a compound molecule can only alter through the addition or subtraction of one or more complete atoms, together with the idea that all the molecules in a pure substance are alike. Fortunately, the compounds at first examined by the chemists engaged in verifying these laws were comparatively simple, so that the whole numbers referred to above were small. The astonishing variety of ratios in which carbon and hydrogen combine was not at first realized. Otherwise Berthollet’s position would have been a much stronger one, and the atomic theory might have had to wait a long while for acceptance. Even at the present time, it would be too much to say that all the complex organic substances have been proved by analysis to obey these laws; all we can assert is that their composition and properties can be satisfactorily explained on the assumption that they do so.
The above statement does not by any means exhaust the possible predictions that can be made from the atomic theory, but it shows how to test the theory. If chemical compounds can be proved by experiment to obey these laws, then the atomic theory acquires a high degree of probability; if they are contradicted by experiment then the atomic theory must be abandoned, or very much modified. Dalton himself made many analyses with the purpose of establishing his views, but his skill as an analyst was not very great. It is in the work of the great Swedish chemist J.J. Berzelius, and somewhat later, in the experiments of the Belgian chemist J.S. Stas, that we find the most brilliant and vigorous verification of these laws, and therefore of the atomic theory.
We shall now give an outline of the experimental evidence for the truth of these laws.
The law of the conservation of matter, an important element in the atomic theory, has been roughly verified by innumerable analyses, in which, a given weight of a substance having been taken, each ingredient in it is isolated Experimental evidence. and its weight separately determined; the total weight of the ingredients is always found to be very nearly equal to the weight of the original substance. But on account of experimental errors in weighing and measuring, and through loss of material in the transfer of substances from one vessel to another, such analyses are rarely trustworthy to more than one part in about 500; so that small changes in weight consequent on the chemical change could not with certainty be proved or disproved. A few experimenters have carried the verification much further. Stas, in his syntheses of silver iodide, weighed the silver and the iodine separately, and after converting them into the compound he weighed this also. In each of a number of experiments he found that the weight of the silver iodide did not differ by one twenty-thousandth of the whole from the sum of the weights of the silver and the iodine used. His analyses of another compound, silver iodate, confirm the law to one part in 78,000. In E.W. Morley’s experiments on the synthesis of water the hydrogen, the oxygen and the water that had been formed were separately determined; taking the mean of his results, the sum of the weights of the ingredients is not found to differ from the weight of the product by one part in 10,000. It is evident that if our experiments are solely directed to the verification of this law, they should, if possible, be carried out in a hermetically closed vessel, the vessel and its contents being weighed before and after the chemical change. The extremely careful experiments of this kind, by H. Landolt and others, made it at first appear that the change in weight, if there is any, consequent on a chemical change can rarely exceed one-millionth of the weight of the reacting substances, and that it must often be much less. The small discrepancies found are so easily accounted for by attributing them to experimental errors that, until recently, every chemist would have regarded the law as sufficiently verified. Landolt’s subsequent experiments showed, what was already noticed in the earlier ones, that these minute changes in weight are nearly always losses, the products weigh less than the components, while if they had been purely experimental errors, due to weighing, they might have been expected to be as frequently gains as losses. Landolt was disposed to attribute these losses in weight to the containing vessel, which was of glass or quartz, not being absolutely impervious, but in 1908 he showed that, by making allowance for the moisture adsorbed on the vessel, the errors were both positive and negative, and were less than one in ten million. He concluded that no change of weight can be detected. Modern researches (see Radioactivity) on the complex nature of the atom have a little shaken the belief in the absolute permanence of matter. But it seems pretty clear that if there is any change in weight consequent on chemical change, it is too minute to be of importance to the chemist, though the methods of modern physics may settle the question. (See Element.)
The law of constant proportions is easily verified to a moderate degree of accuracy by such experiments as the following. We can prepare, in the laboratory, a white powder that proves to be calcium carbonate, that is, it appears to be wholly composed of carbon dioxide and lime. We find in nature two other unlike substances, marble and Iceland spar, each of which is wholly composed of carbon dioxide and lime. Thus these three substances, unlike in appearance and origin, are composed of the same ingredients: if small variations in the combining ratio of the components were possible, we might expect to find them in such a case as this. But analysis has failed to find such differences; the ratio of the weights of lime and carbon dioxide is found to be the same in all three substances. Such analyses, which do not always admit of great accuracy, have been confirmed by a few carefully planned experiments in which two components were brought together under very varied conditions, and the resulting compound analysed. Stas carried out such experiments on the composition of silver chloride and of ammonium chloride, but he never found a variation of one part in 10,000 in the composition of the substances.
The two laws discussed above were more or less accepted before the promulgation of the atomic theory, but the law of multiple proportions is the legitimate offspring of this theory. Berzelius saw at once that it afforded an admirable test for the correctness of Dalton’s views, and he made numerous experiments expressly designed to test the law. One of these experiments may be described. Two chlorides of copper are known, one a highly coloured substance, the other quite white. Berzelius took 8 grams of copper, converted it into the coloured chloride, and sealed up the whole of this in solution, together with a weighed strip of copper. After some time the colour entirely disappeared; the strip of copper was then taken out and reweighed, and it was found to have lost 8.03 grams. Thus the chlorine, which in the coloured compound was in union with 8 grams of copper, appears, in the colourless chloride, to be combined with 16.03 grams, or almost exactly double the amount. It is easy to verify this result. In a series of repetitions of the experiment, by different observers, the following numbers were obtained for the ratio of the copper in the two chlorides: 1.98, 1.97, 2.03, 2.003, the mean value being 1.996. It will be noticed that the ratio found is sometimes above and sometimes below the number 2, which is required by the atomic theory, and therefore the deviations may not unreasonably be attributed to experimental errors. Such experiments—and numerous ones of about this degree of accuracy have been made on a variety of substances—give a high degree of probability to the law, but leave it an open question whether it has the exactitude of the law of the conservation of matter, or whether it is only approximately true. The question is, however, vital to the atomic theory. It is, therefore, worth while to quote a verification of great exactitude from the work of Stas and J.B.A. Dumas3 on the composition of the two oxides of carbon. From their work it follows that the ratio of the weights of oxygen combined with unit weight of carbon in the two oxides is 1.99995, or with somewhat different data, 1.9996.
The law of reciprocal proportion, of which some examples have been already given, is part of a larger law of equivalence that underlies most of our chemical methods and calculations. One section of the law expresses the fact that the weights of two substances, not necessarily elements, that are equivalent in one reaction, are often found to be equivalent in a number of other reactions. The neutralization of acids by bases affords many illustrations, known even before the atomic theory, of the truth of the statement. It is universally found that the weights of two bases which neutralize the same weight of one acid are equivalent in their power of neutralizing other acids. Thus 5 parts by weight of soda, 7 of potash and 3.5 of quicklime will each neutralize 4.56 parts of hydrochloric acid or 7.875 of nitric or 6.125 parts of sulphuric acid; these weights, in fact, are mutually equivalent to one another. The Daltonian would say that each of these weights represents a certain group of atoms, and that these groups can replace, or combine with, each other, to form new molecules. The change from a binary compound, that is, one containing two elements, to a ternary compound in which these two elements are associated with a third, sometimes affords a very good test for the theory. The atomic theory can picture the change from the binary to the ternary compound simply as the addition of one or more atoms of the third element to the previously existing molecule; in such a case the combining ratio of the first two elements should be absolutely the same in both compounds. Berzelius tested this prediction. He showed that lead sulphide, a black substance containing only lead and sulphur, could be converted by oxidation into lead sulphate, a white compound containing oxygen as well as lead and sulphur. The whole of the lead and sulphur of the sulphide was found to be present in the sulphate; in other words, the combining ratio of the lead and sulphur was not altered by the addition of the oxygen. This is found to be a general rule. It was verified very exactly by Stas’s experiments, in which he removed the oxygen from the ternary compound silver iodate and found that the whole of the silver and the iodine remained in combination with each other as silver iodide; his results prove, to one part in ten millions, that the combining ratio of the silver and the iodine is unaltered by the removal of the oxygen.
The above gives some idea of the evidence that has been accumulated in favour of the laws of chemical combination, laws which can be deduced from the atomic theory. Whenever any of these laws, or indeed any prediction from the theory, can be tested it has so far proved to be in harmony with experiment. The existence of the periodic law (see Element), and the researches of physicists on the constitution of matter (q.v.), also furnish very strong support to the theory.
Dalton was of the opinion that it was possible to determine the weights of the elementary atoms in terms of any one by the analysis of compounds. It is evident that this is practicable if the number and kind of atoms contained Atomic weight. in the molecule of a compound can be determined. To take the simplest possible case, if Dalton had been correct in assuming that the molecule of water was made up of one atom of oxygen and one of hydrogen, then the experimental fact that water contains eight parts by weight of oxygen to one part of hydrogen, would at once show that the atom of oxygen is eight times as heavy as the atom of hydrogen, or that, taking the atomic weight of hydrogen as the unit, the atomic weight of oxygen is 8. Similarly, Dalton’s diagram for ammonia, together with the fact that ammonia contains 4.67 parts of nitrogen to one of hydrogen, at once leads to the conclusion that the atomic weight of nitrogen is 4.67. But, unfortunately, the assumption as to the number of atoms in the molecules of these two compounds was an arbitrary one, based on no valid evidence. It is now agreed that the molecule of water contains two atoms of hydrogen and one of oxygen, so that the atomic weight of oxygen becomes 16, and similarly that the molecule of ammonia contains three atoms of hydrogen and one of nitrogen, and that consequently the atomic weight of nitrogen is 14. On account of this difficulty, the atomic weights published by Dalton, and the more accurate ones of Berzelius, were not always identical with the values now accepted, but were often simple multiples or submultiples of these.
The “symbols” for the elements used by Dalton, apparently suggested by those of the alchemists, have been rejected in favour of those which were introduced by Berzelius. The latter employed the first letter, or the first two letters, Formulae. of the name of an element as its symbol. The symbol, like that of Dalton, always stands for the atomic weight of the element, that is, while H stands for one part by weight of hydrogen, O stands for 16 parts of oxygen, and so on. The symbols of compounds become very concise, as the number of atoms of one kind in a molecule can be expressed by a sub-index. Thus the symbol or formula H2O for water expresses the view that the molecule of water consists of one atom of oxygen and two of hydrogen; and if we know the atomic weights of oxygen and hydrogen, it also tells us the composition of water by weight. Similarly, the modern formula for ammonia is NH3.
The superiority of this notation over that of Dalton is not so obvious when we consider such simple cases as the above, but chemists are now acquainted with very complex molecules containing numerous atoms; cane sugar, for example, has the formula C12H22O11. It would be a serious business to draw a Daltonian diagram for such a molecule.
Dalton believed that the molecules of the elementary gases consisted each of one atom; his diagram for hydrogen gas makes the point clear. We now believe that the molecule of an element is frequently made up of two or more atoms; thus the formulae for the gases hydrogen, oxygen and nitrogen are H2, O2, N2, while gaseous phosphorus and sulphur are probably P4 and S6, and gaseous mercury is Hg1,—that is, the molecule of this element is monatomic. This view, as to the frequently complex nature of the elementary molecule, is logically and historically connected with the striking hypothesis of Amadeo Avogadro and A.M. Ampère. These natural philosophers suggested that equal volumes of all gaseous substances must contain, at the same temperature and pressure, the same number of molecules. Their hypothesis explains so many facts that it is now considered to be as well established as the parts of the theory due to Dalton.4 This principle at once enables the weights of molecules to be compared even when their composition is unknown; it is only necessary to determine the specific gravities of the various gases referred to some one of them, say hydrogen; the numbers so obtained giving the weights of the molecules referred to that of the hydrogen molecule.
The atomic theory has been of priceless value to chemists, but it has more than once happened in the history of science that a hypothesis, after having been useful in the discovery and the co-ordination of knowledge, has been abandoned Present position of the atomic theory. and replaced by one more in harmony with later discoveries. Some distinguished chemists have thought that this fate may be awaiting the atomic theory, and that in future chemists may be able to obtain all the guidance they need from the science of the transformations of energy. But modern discoveries in radioactivity5 are in favour of the existence of the atom, although they lead to the belief that the atom is not so eternal and unchangeable a thing as Dalton and his predecessors imagined, and in fact, that the atom itself may be subject to that eternal law of growth and decay of which Lucretius speaks.
1 Robert Boyle, The Sceptical Chymist (1661); The Usefulness of Natural Philosophy (1663).
2 Sir Isaac Newton, Principia, bk. ii. prop. 23.
3 Freund, The Study of Chemical Composition.
4 It will be seen that in the three gas diagrams of Dalton that are reproduced above, equal numbers of molecules are contained in equal volumes, but if Dalton held this view at one time he certainly afterwards abandoned it.
5 Rutherford, Radioactivity.


